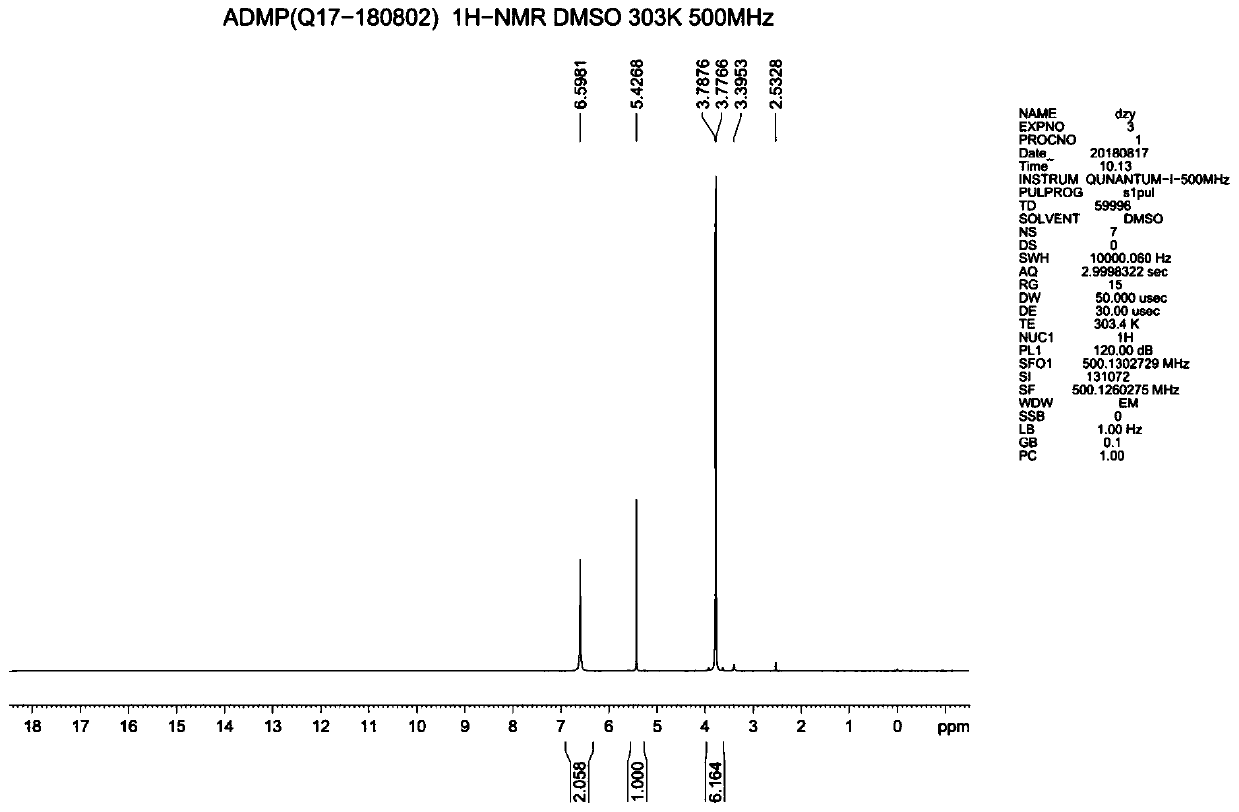
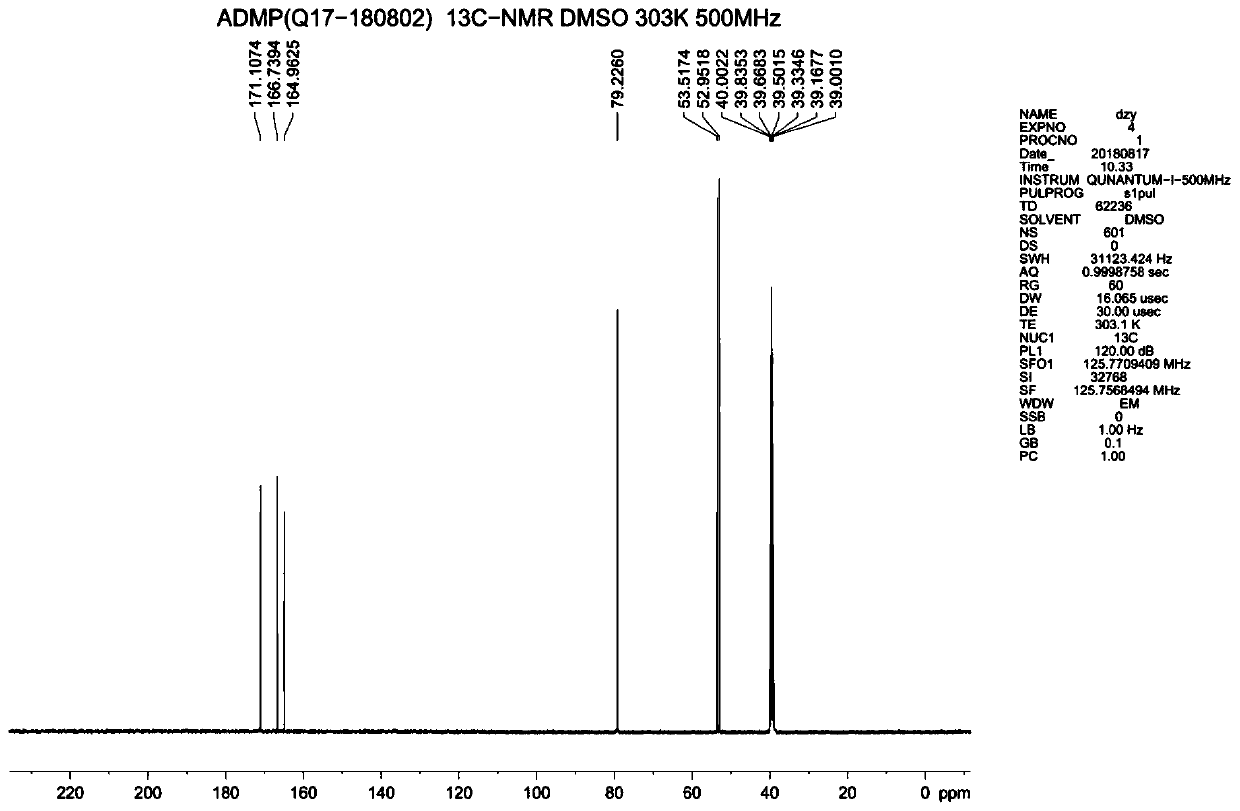
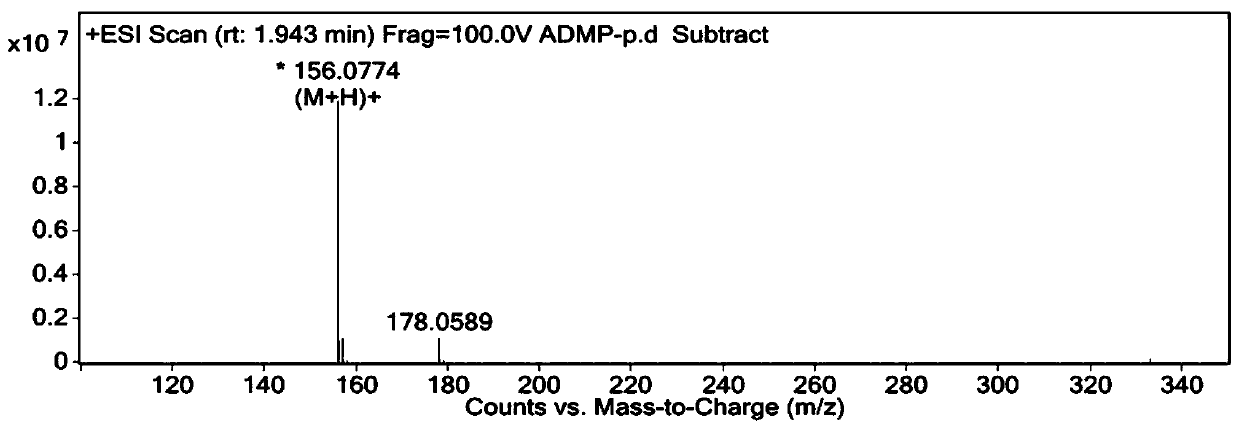
Synthesis method of 4-amino-2,6-dimethoxyl pyrimidine
A technique for the synthesis of dimethoxypyrimidine and its synthesis method, which is applied in the field of synthesis of 4-amino-2.6-dimethoxypyrimidine, and can solve the problems of low yield in the chlorination step, increased process risk, and a large amount of phosphorous-containing wastewater. , to achieve the effects of less environmental pollution, avoiding emissions, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprises the steps:
[0032] (1) Add 300ml of water and 26.5g of 28% ammonia water into a 500mL four-necked reaction flask, add 40g of 4,6-dichloropyrimidine-5-carboxylic acid under stirring, then keep warm at 30°C and react for 6 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0033] (2) After the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-formic acid to 3 with hydrochloric acid, pass into about 15g of chlorine gas, control the temperature during the process of flowing chlorine gas between 10~40°C, and end the flow of chlorine gas, Use liquid caustic soda to adjust the pH to 4-5, and filter below 20°C to obtain the wet product of 4-amino-2,6-dichloropyrimidine-5-carboxylic acid;
[0034] (3) Add 1000ml of water into a 2L pressure reactor, put in the 4-amino-2,6-dichloropyrimidine-5-carboxylic acid obtained in step (2), raise the temperature to 150°C, react for 2 hour...
Embodiment 2
[0037] A kind of synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprises the steps:
[0038] (1) Add 300ml of water and 32.7g of 28% ammonia water into a 500mL four-necked reaction flask, add 40g of 4,6-dichloropyrimidine-5-carboxylic acid under stirring, then keep warm at 50°C and react for 12 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0039] (2) After the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-formic acid to 3 with hydrochloric acid, pass into about 15g of chlorine gas, control the temperature during the process of flowing chlorine gas between 10~40°C, and end the flow of chlorine gas, Use liquid caustic soda to adjust the pH to 4-5, and filter below 20°C to obtain the wet product of 4-amino-2,6-dichloropyrimidine-5-carboxylic acid;
[0040] (3) Add 1000ml of water into a 2L pressure reactor, put in the wet product of 4-amino-2,6-dichloropyrimidine-5-carboxylic acid obtained in step (2), raise the temperature to 110°C, ...
Embodiment 3
[0043] A kind of synthetic method of 4-amino-2.6-dimethoxypyrimidine, comprises the steps:
[0044] (1) Add 300ml of water and 32.7g of 28% ammonia water into a 500mL four-necked reaction flask, add 40g of 4,6-dichloropyrimidine-5-carboxylic acid under stirring, then keep warm at 50°C and react for 12 hours to obtain 4-amino- 6-chloropyrimidine-5-carboxylic acid;
[0045](2) After the reaction finishes, adjust the pH of 4-amino-6-chloropyrimidine-5-formic acid to 3 with hydrochloric acid, pass into about 15g of chlorine gas, control the temperature during the process of flowing chlorine gas between 10~40°C, and end the flow of chlorine gas, Use liquid caustic soda to adjust the pH to 4-5, and filter below 20°C to obtain the wet product of 4-amino-2,6-dichloropyrimidine-5-carboxylic acid;
[0046] (3) Add 1000ml of water into a 2L pressure reactor, put in the wet product of 4-amino-2,6-dichloropyrimidine-5-carboxylic acid obtained in step (2), raise the temperature to 110°C, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com