Gonadotropin-releasing hormone antagonist dosing regimens for the treatment of endometriosis
A technology of endometrium and antagonists, which is applied in the direction of pharmaceutical formulations, diseases, drug combinations, etc., and can solve problems such as adverse side effects, receptor insensitivity, and restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0572] Example 1. Determination of initial doses of GnRH antagonists for the treatment of endometriosis in human subjects based on AMH levels
[0573] Using the methods of the present invention, a clinician skilled in the art can assess a patient's AMH levels prior to initiation of GnRH antagonist therapy for the treatment of endometriosis. The GnRH antagonist to be administered to the patient may be a compound represented by formula (III), namely choline 3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)4 -Methoxyphenyl]-2,4-dioxo-1,2,3,4-tetrahydrothieno[3,4d]pyrimidine-5-carboxylate. A clinician skilled in the art can draw blood from a patient and can then perform one or more analytical techniques, such as immunoassays known in the art or described herein, to determine the concentration of AMH in a blood sample isolated from the patient. Quantitative. If it is determined that the concentration of AMH in the sample is elevated relative to the AMH reference range of 15pM to 35...
Embodiment 2
[0577] Example 2. Dosage titration of GnRH antagonists for the treatment of endometriosis in human subjects based on E2 levels
[0578] Using the methods of the present invention, a clinician skilled in the art can assess E2 levels in a patient currently undergoing GnRH antagonist therapy for the treatment of endometriosis in order to determine whether the same dose of GnRH antagonist should continue to be administered to the patient. agent, or whether different doses of a GnRH antagonist should be administered to the patient in order to be effective in reducing one or more symptoms of endometriosis. The GnRH antagonist to be administered to the patient may be a compound represented by formula (III), namely choline 3-[2-fluoro-5-(2,3-difluoro-6-methoxybenzyloxy)4 -Methoxyphenyl]-2,4-dioxo-1,2,3,4-tetrahydrothieno[3,4d]pyrimidine-5-carboxylate. A clinician skilled in the art can draw blood from a patient and can then perform one or more analytical techniques, such as immunoass...
Embodiment 3
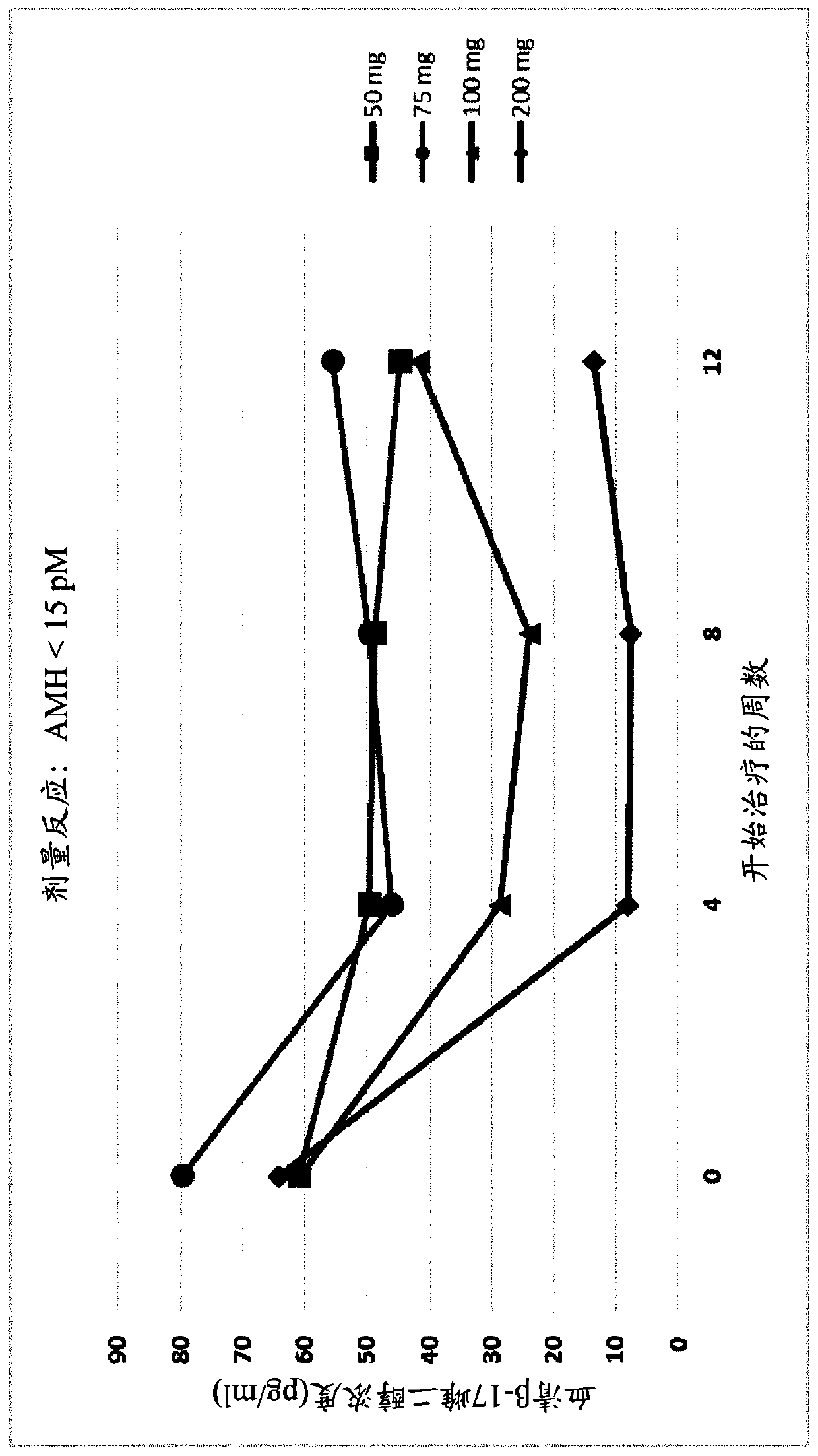
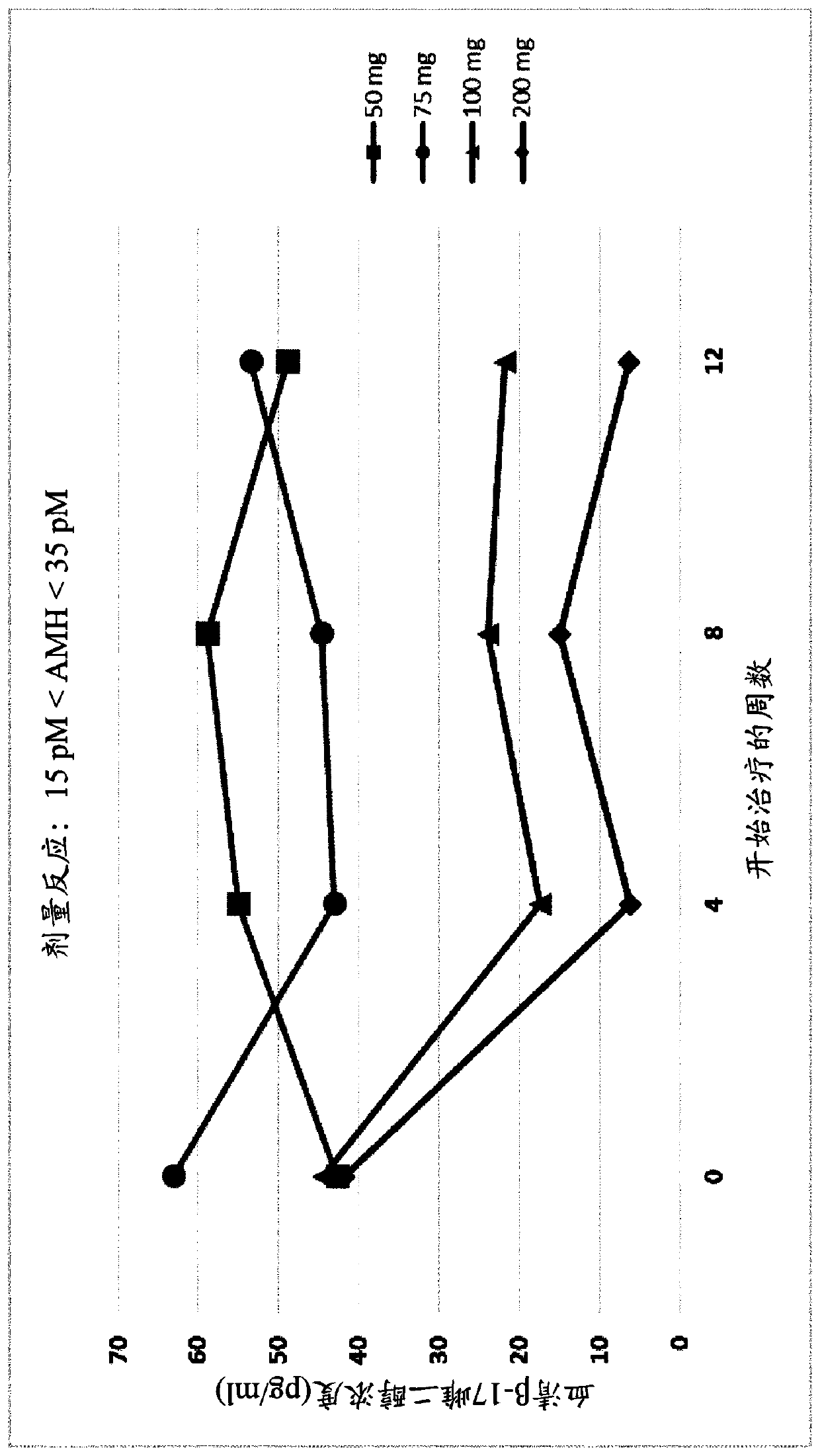
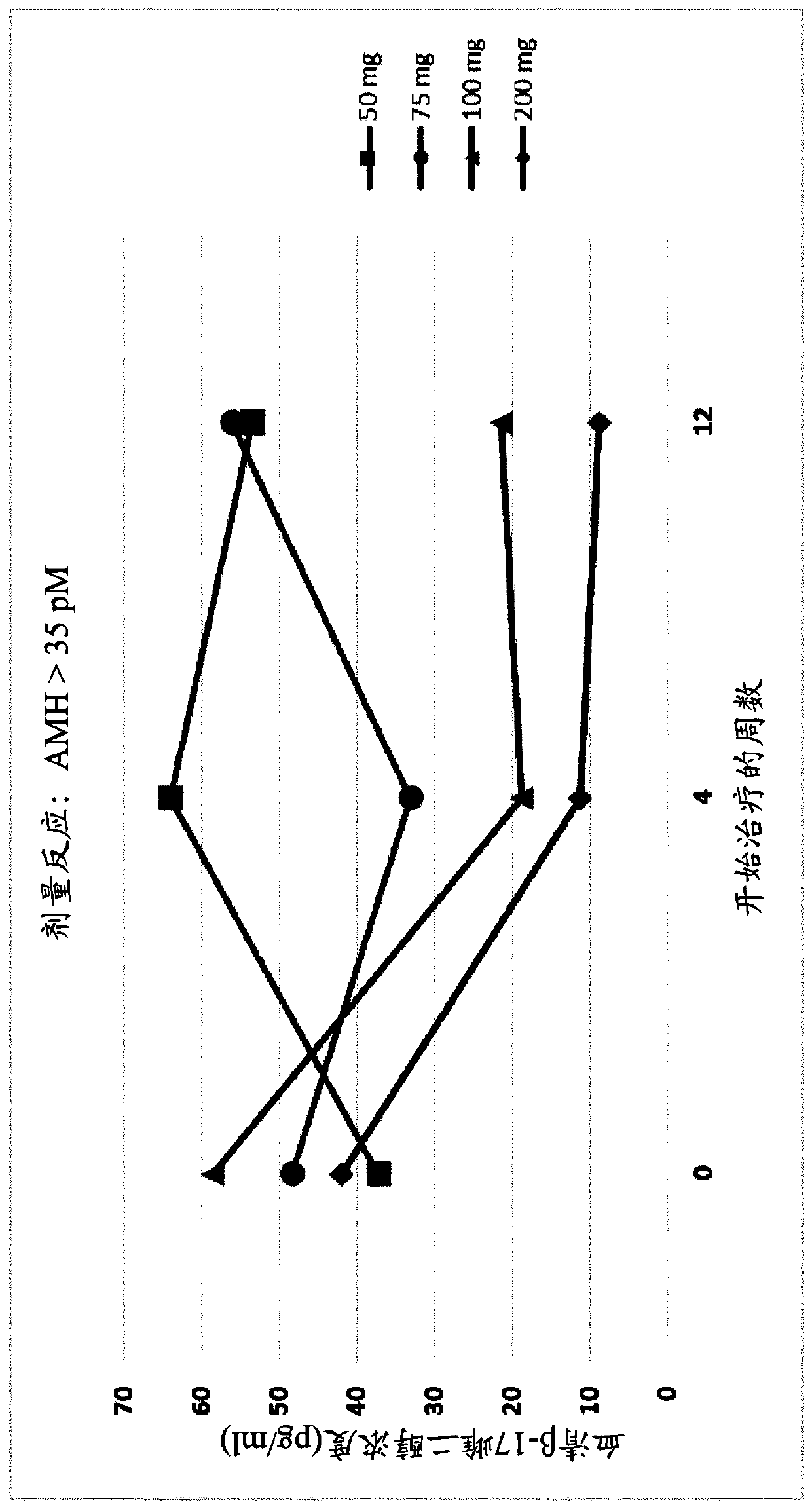
[0582] Example 3. Administration of GnRH Antagonists to Endometriosis Patients Presenting Different AMH Levels
[0583] To study the effect of GnRH antagonists, such as compounds (I), (II), and their pharmaceutically acceptable salts, such as their choline salt (compound (III)) on endometriosis exhibiting different initial values of serum AMH To determine the effect on patients with the disease, a series of experiments were carried out in which the patients were divided into treatment groups and compound (III) or placebo was administered orally. Specifically, a series of n=327 female human patients with endometriosis were divided into five groups and received placebo, 50 mg / day of Compound (III), 75 mg / day of Compound (III) by oral delivery ), 100 mg / day of Compound (III) or 200 mg / day of Compound (III). Treatment with placebo or compound (III) at the doses specified above was continued over a period of 12 weeks.
[0584] At various time points throughout these experiments...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com