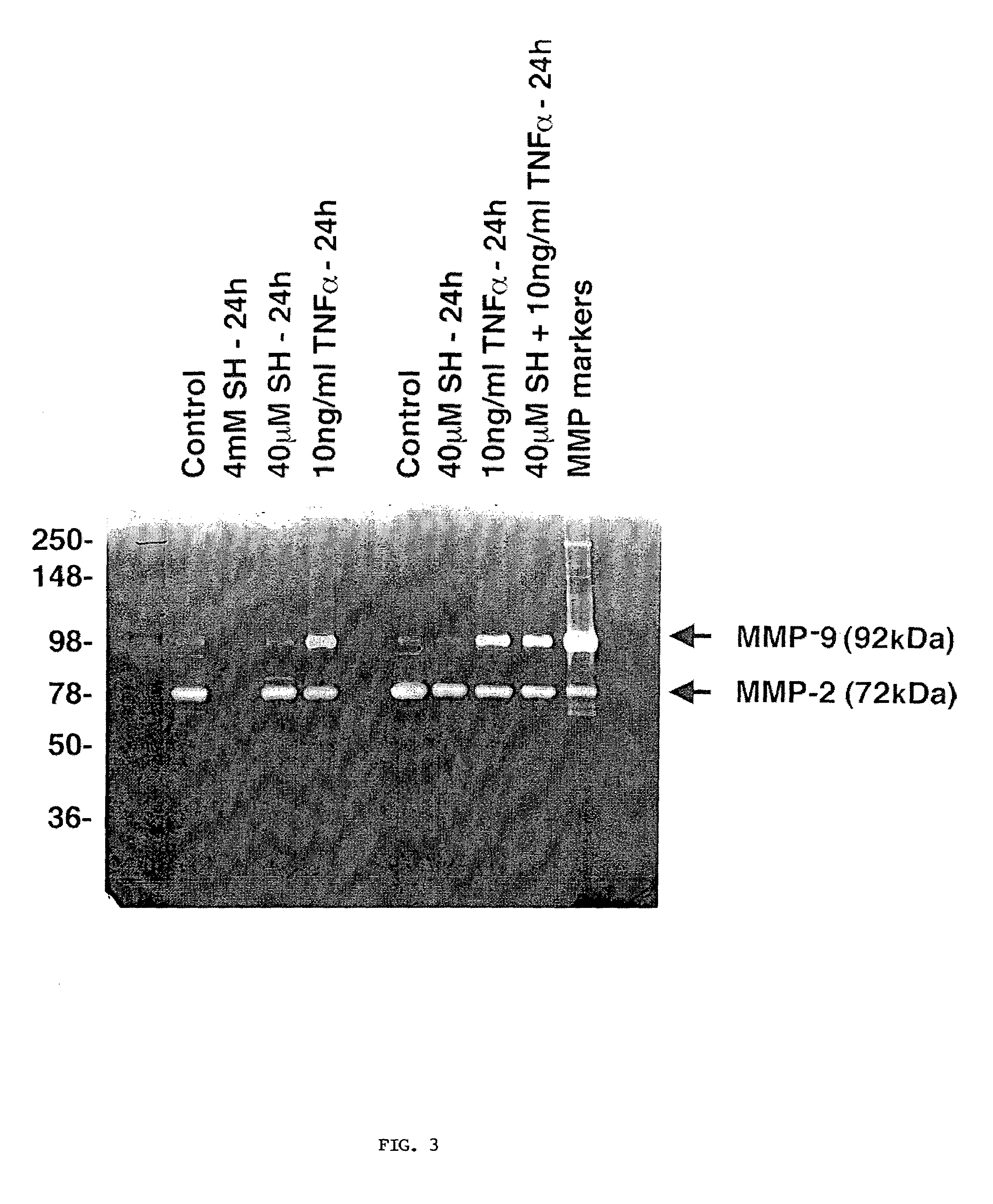
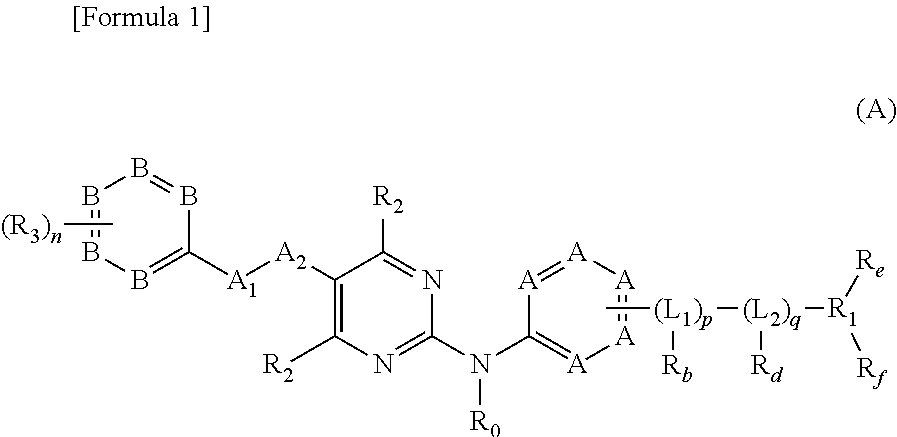
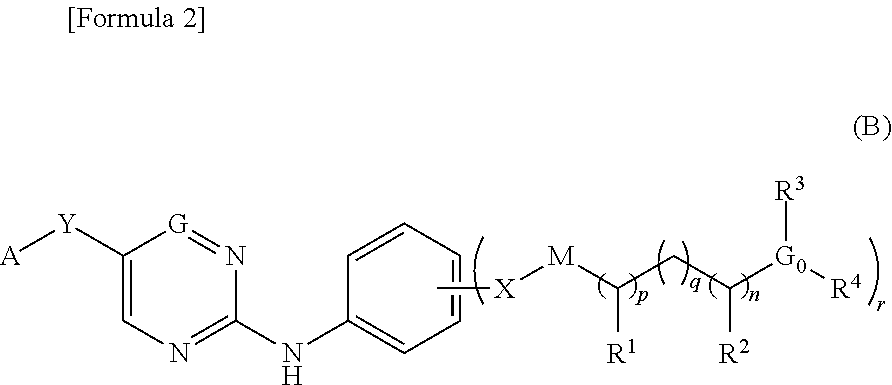
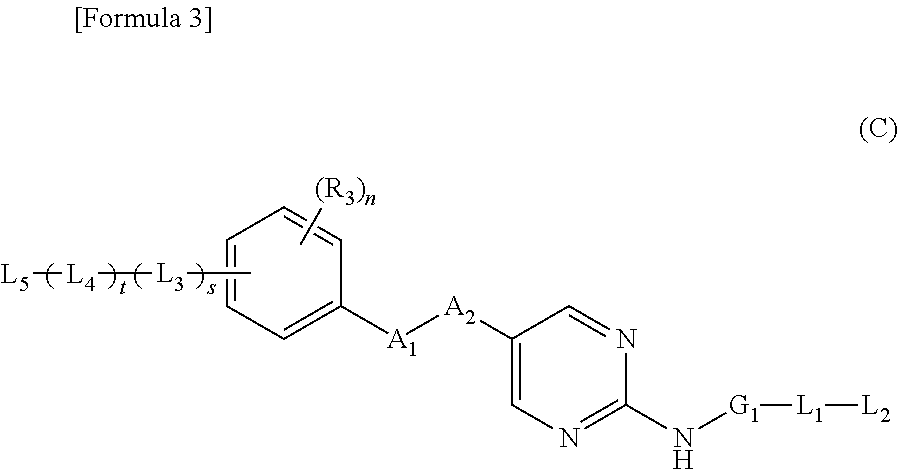
Patents
Literature
61 results about "Hormone therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hormone therapy or hormonal therapy is the use of hormones in medical treatment. Treatment with hormone antagonists may also be referred to as hormonal therapy or antihormone therapy. The most general classes of hormone therapy are oncologic hormone therapy, hormone replacement therapy (for menopause), androgen replacement therapy (ART), and transgender hormone therapy.
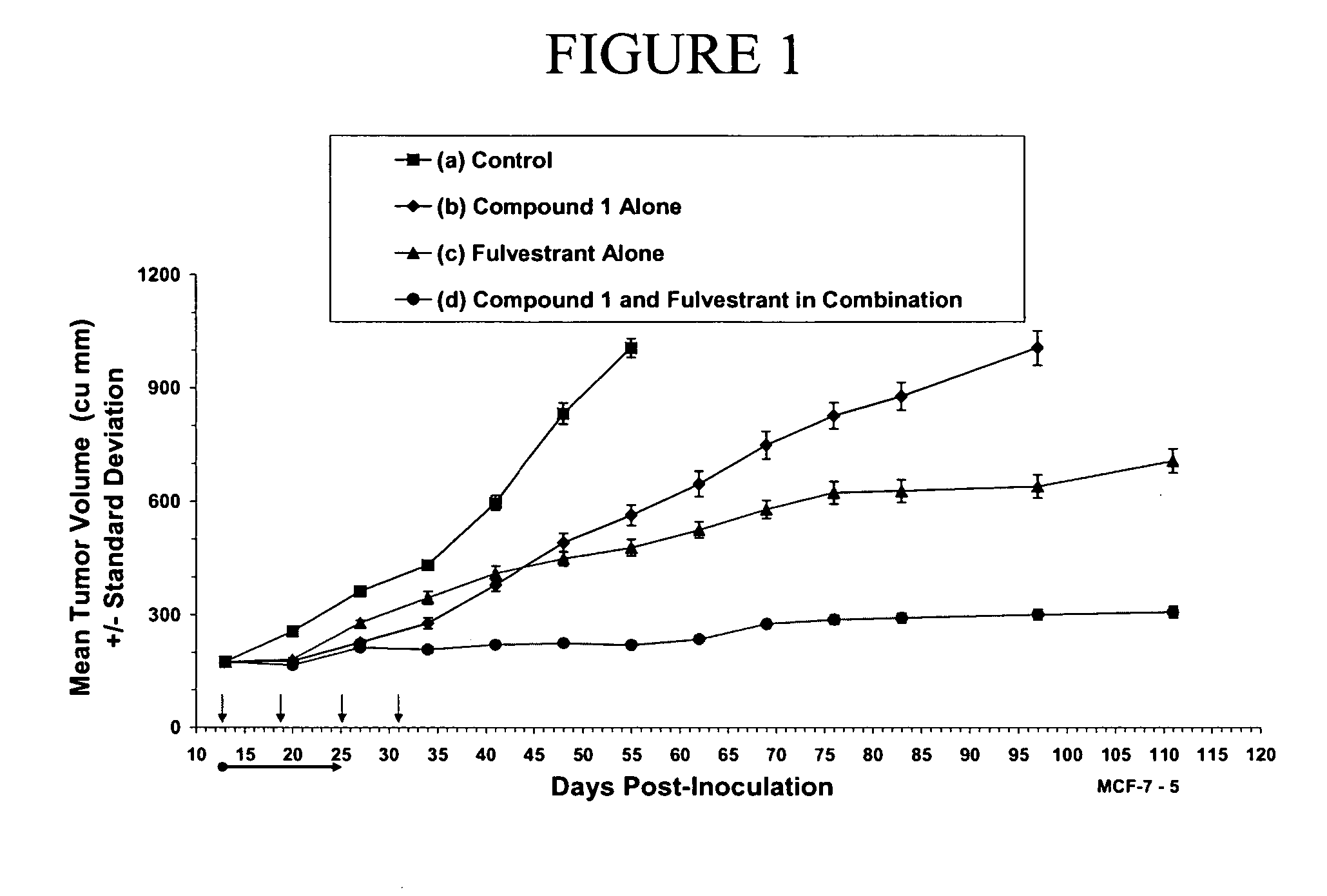
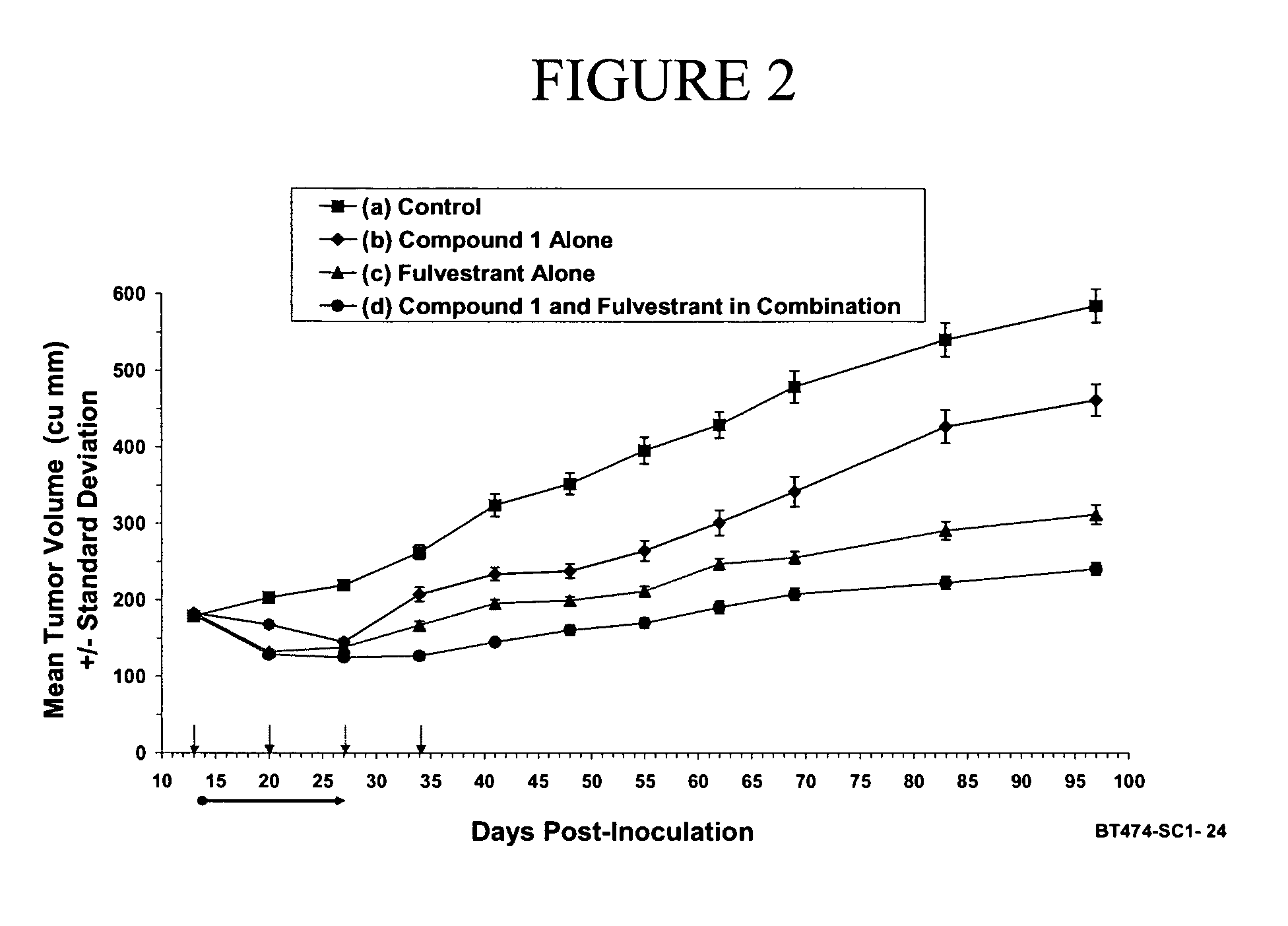
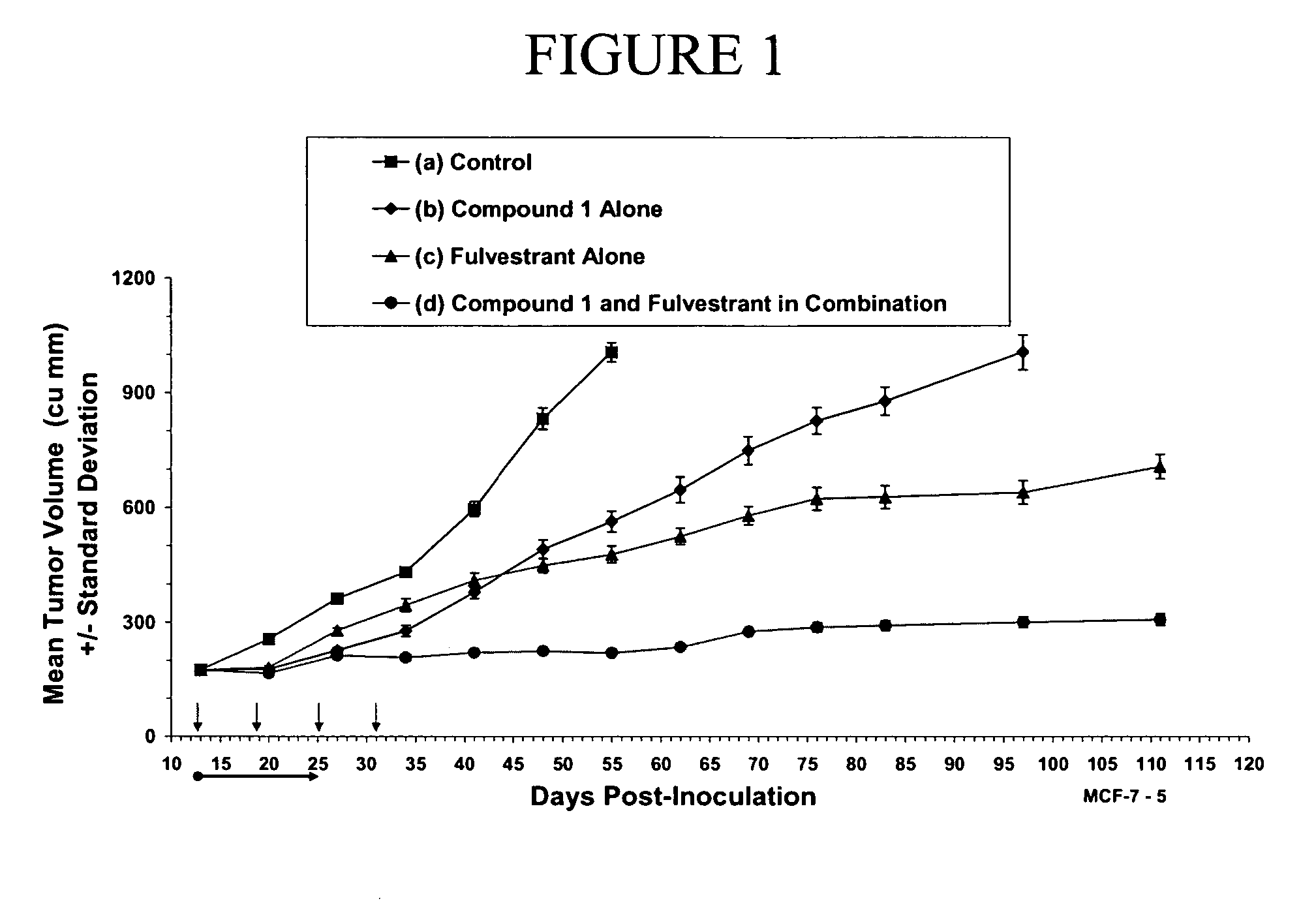
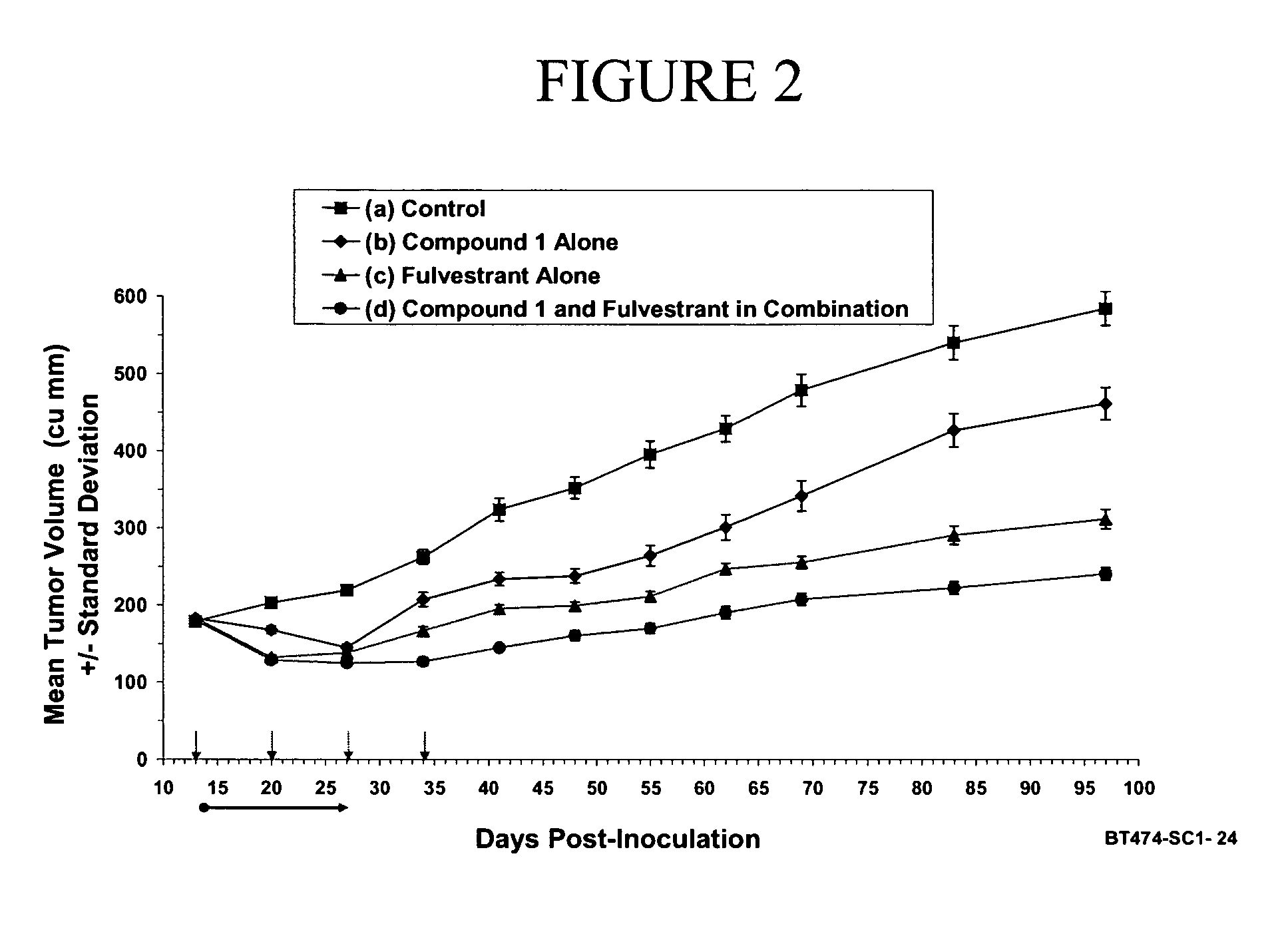
Combination therapy for the treatment of cancer
The present invention provides methods of treating cancer comprising treating a patient in need thereof with hormone therapy and administering to the patient a therapeutically effective amount of at least one metalloprotease inhibitor. The present invention also provides pharmaceutical compositions comprising a therapeutically effective amount of at least one hormone therapy agent, at least one metalloprotease inhibitor, and a pharmaceutically acceptable carrier.
Owner:INCYTE HLDG CORP
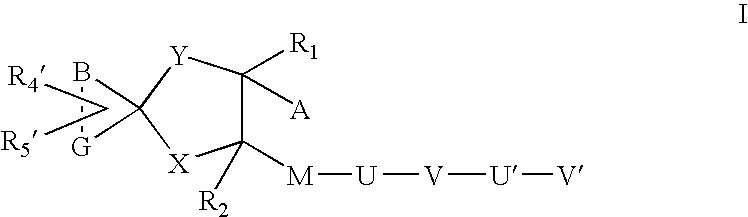
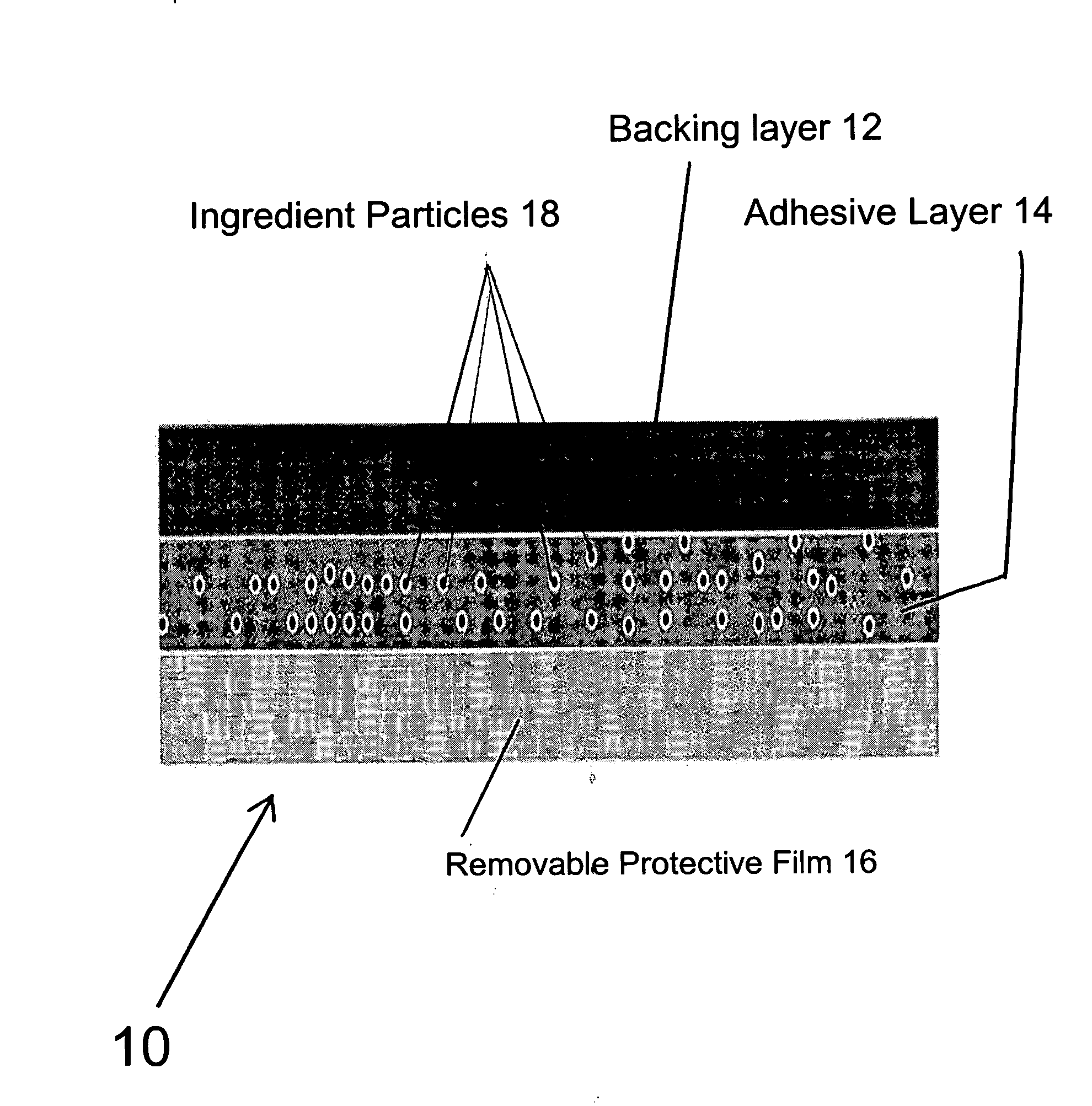
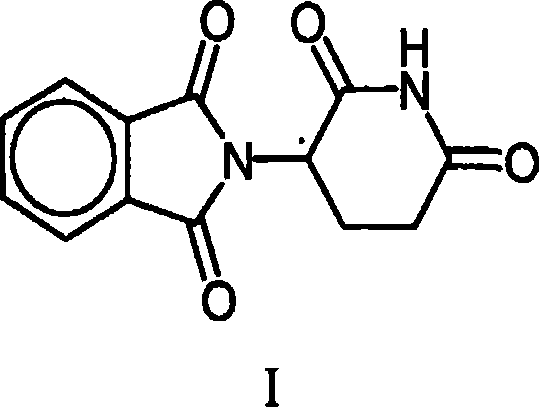
Transdermal pharmaceutical preparation with a progesterone A-specific ligand (PRASL) as active ingredient
InactiveUS20060134188A1Avoid reactionReduce riskBiocideOrganic active ingredientsTransdermal patchAdditive ingredient
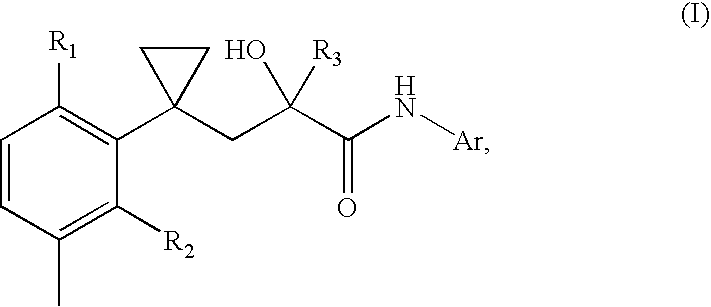
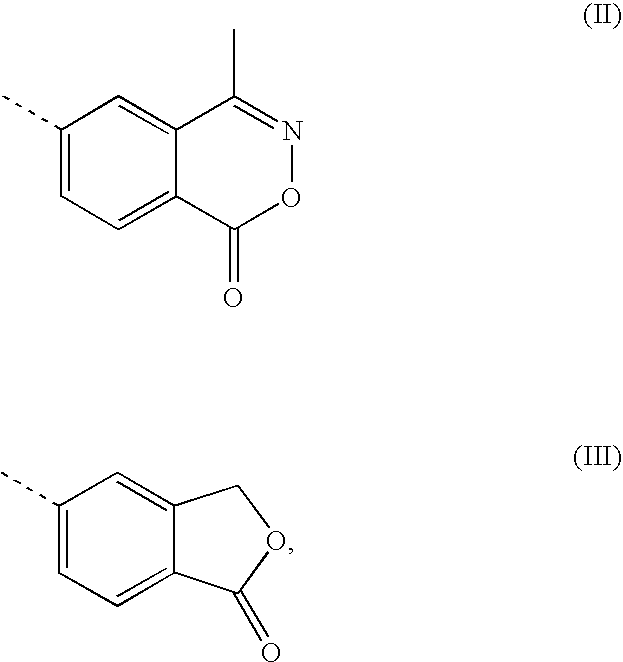
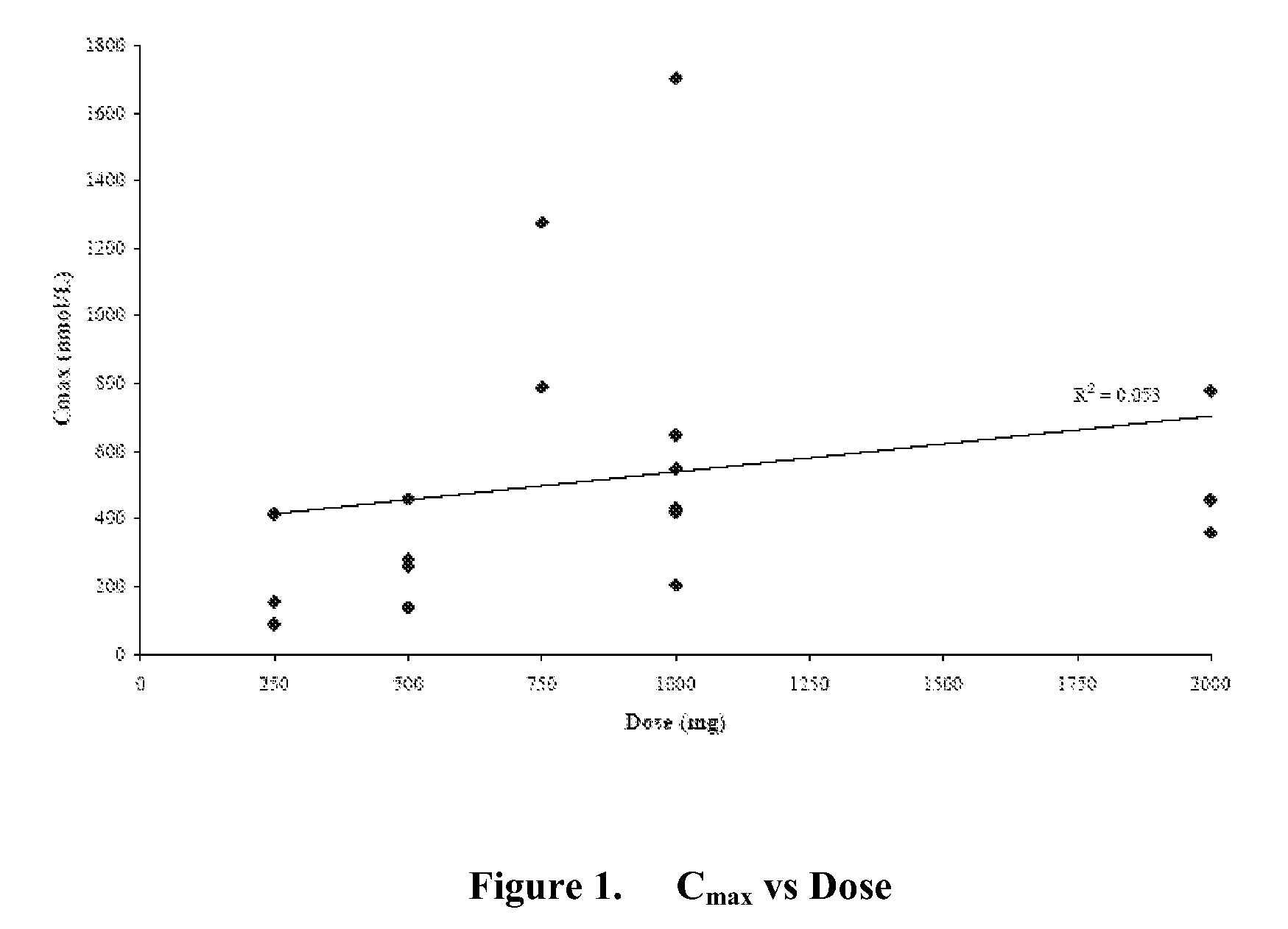
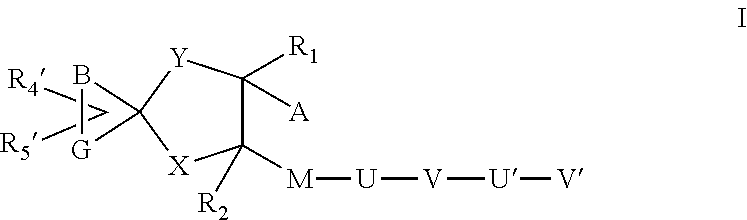
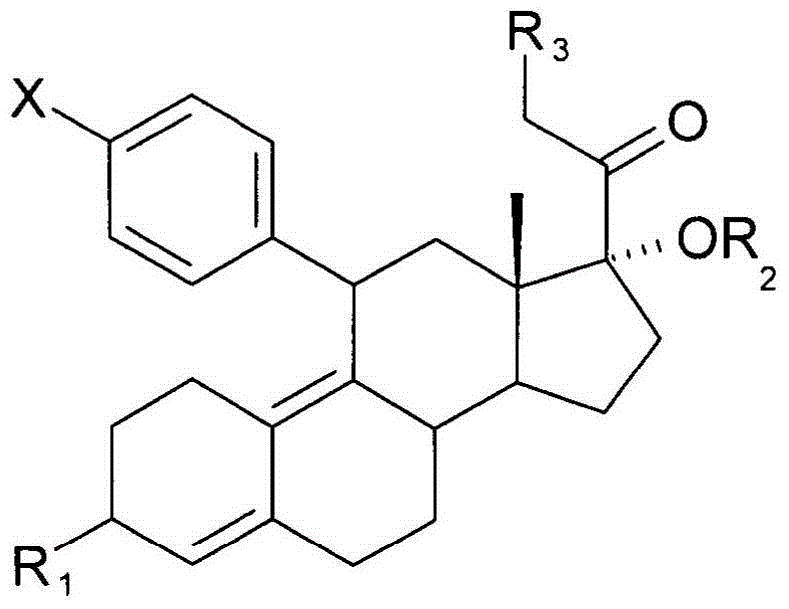
A transdermal patch for hormone therapy and fertility control has a backing layer, an effective-ingredient-containing adhesive layer adhering to the backing layer and a removable protective film. The adhesive layer includes a progestagenic effective ingredient and an estrogen in an adhesive matrix based on a silicone polymer, a polyisobutylene polymer (PIB), a polyacrylate polymer or a styrene block copolymer with butadiene or isoprene (SBS or SIS). The transdermal patch contains from 0.1 to 10%, based on a total weight of the adhesive matrix, of a progestagenic effective ingredient of formula I: wherein R1 and R2 each represent, independently of each other, H or F; R3 represents CH3 or CF3 and Ar is a group of formula II or III: or a pharmaceutically suitable derivative thereof.
Owner:SCHERING AG
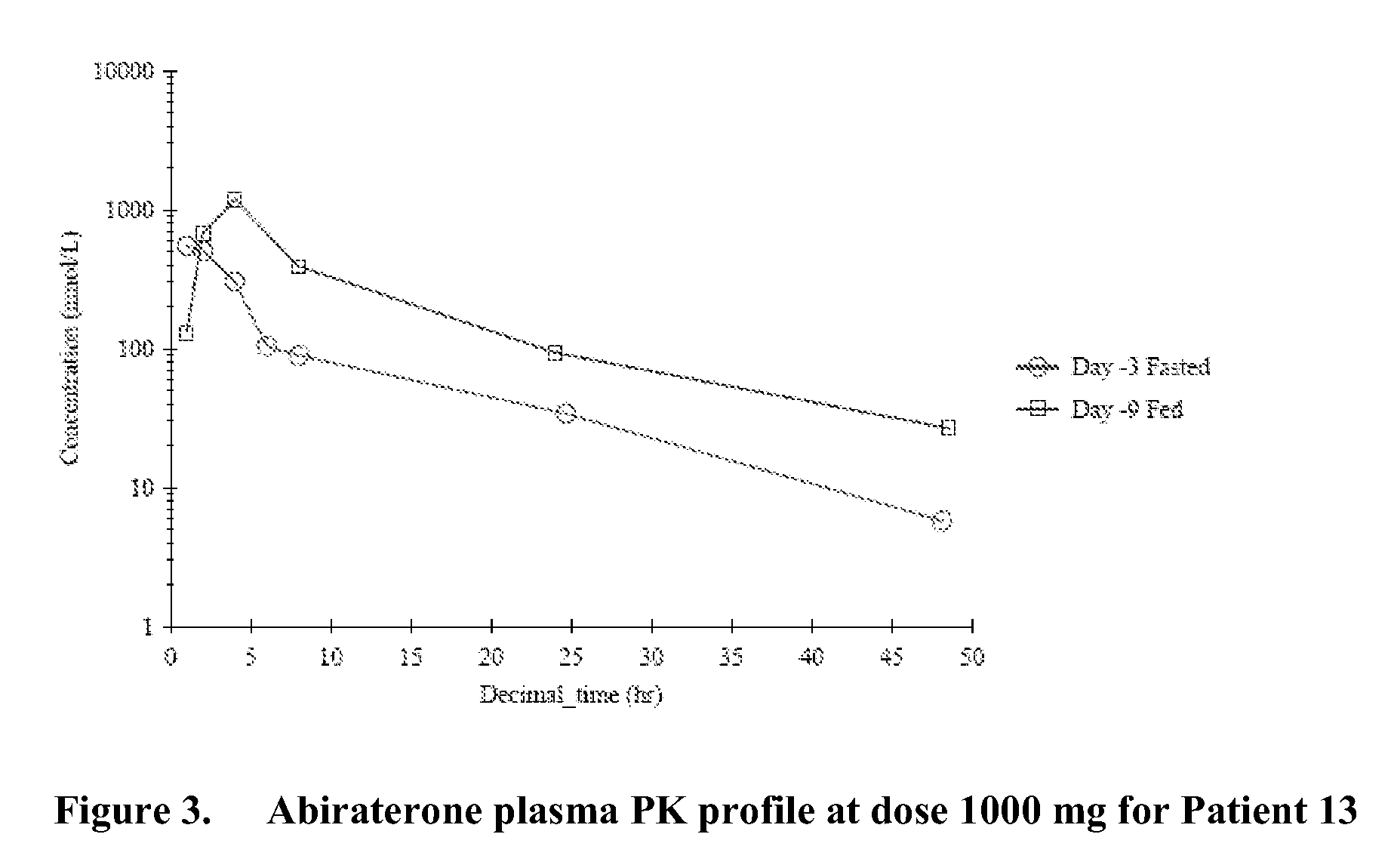
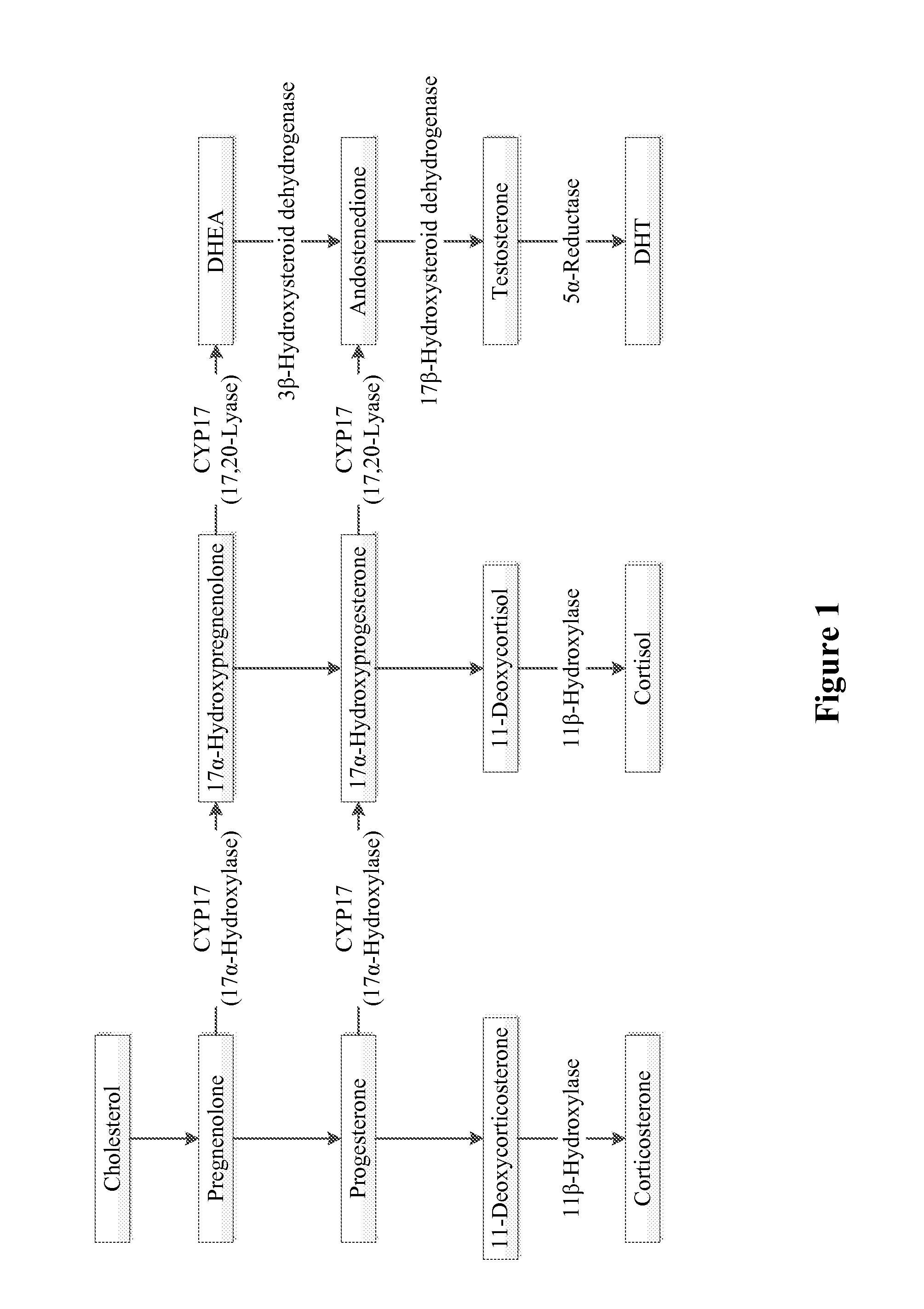
METHODS FOR TREATING CANCER USING 17alpha-HYDROXYLASE/C17,20-LYASE INHIBITORS
InactiveUS20090124587A1Reducing and avoiding progressReduced plasma concentrationOrganic active ingredientsAntineoplastic agentsMetaboliteRegimen
Owner:AUERBACH ALAN H +1
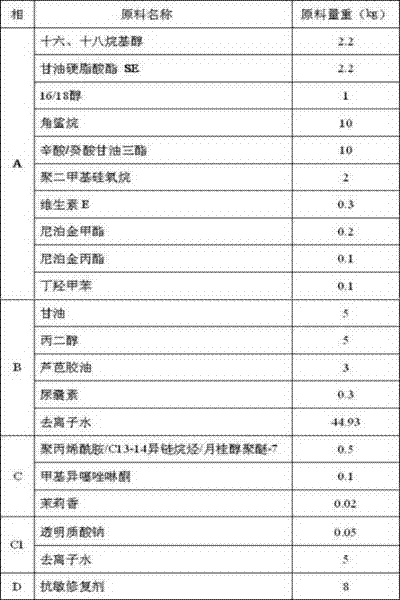
Anti-sensitization and restoration external preparation for skin
ActiveCN102342993AGrowth inhibitionInhibit growthCosmetic preparationsAnthropod material medical ingredientsAllergic dermatitisSide effect
The invention relates to an anti-sensitization and restoration external preparation for skin, belonging to the technical field of Chinese medicinal preparations. Based on the research and treatment principle of the pathogenic mechanism of skin impairment aggravation of a patient caused by sensitive or allergic dermatitis and hormone therapy sensitive or allergic dermatitis according to the traditional Chinese medicine and in combination with other component raw materials, active ingredients contained in the preparation have the effects of clearing away heat and toxic materials, resisting sensitization, restoring, converging and increasing skin elasticity and luster. The preparation can be prepared into cream or water aqua. The anti-sensitization and restoration external preparation is directly applied to the infection focuses of the patient, has no toxic or side effect, has a good curative effect, is easy to use, and is suitable for the patient suffering from skin impairment caused bysensitive or allergic dermatitis and hormone therapy sensitive or allergic dermatitis; and the problems of larger skin impairment of the patient caused by the conventional hormone therapy of sensitive or allergic dermatitis, difficulty in treating and poor curative effect are solved.
Owner:广州市康蓉科技有限公司
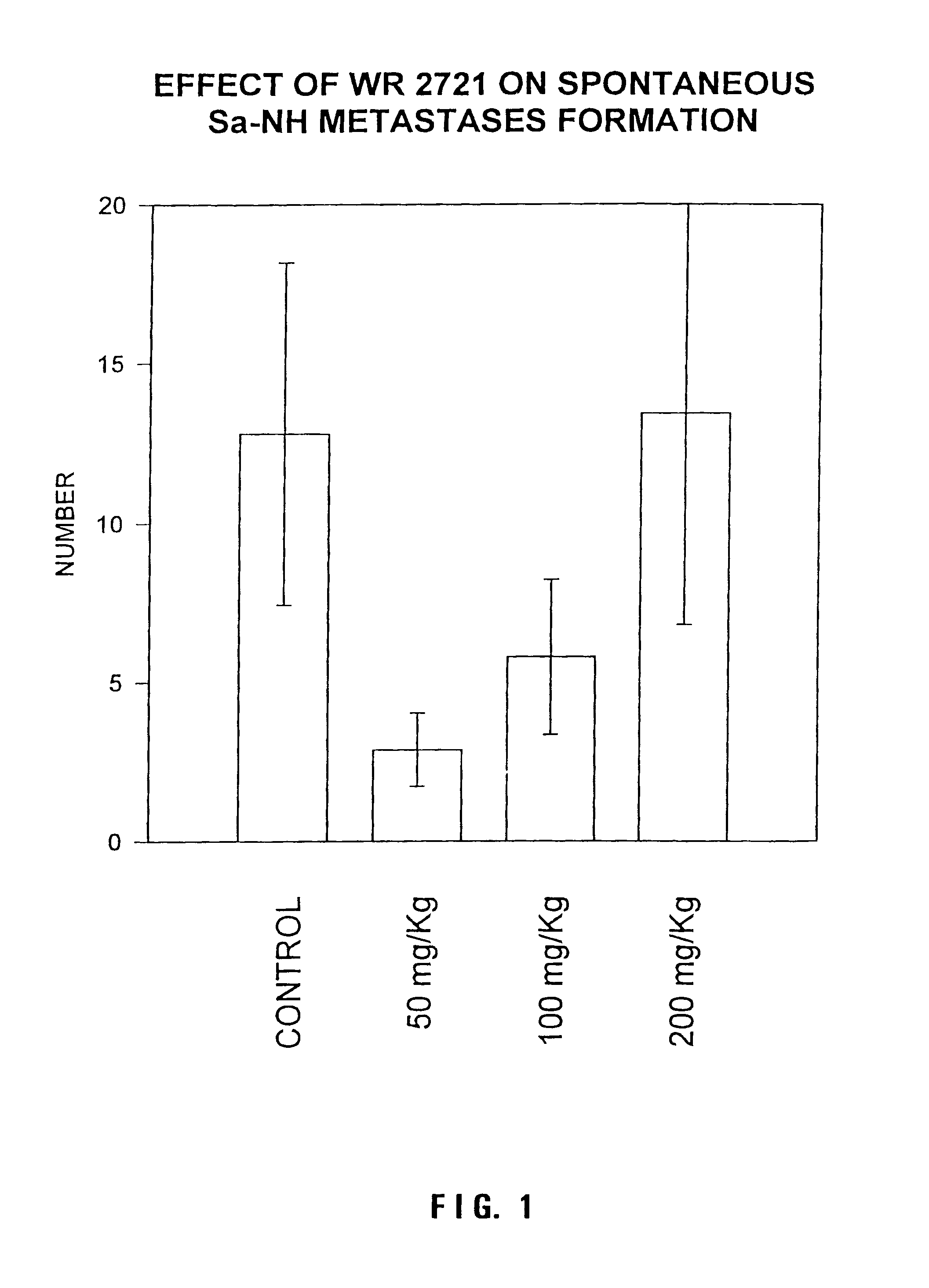
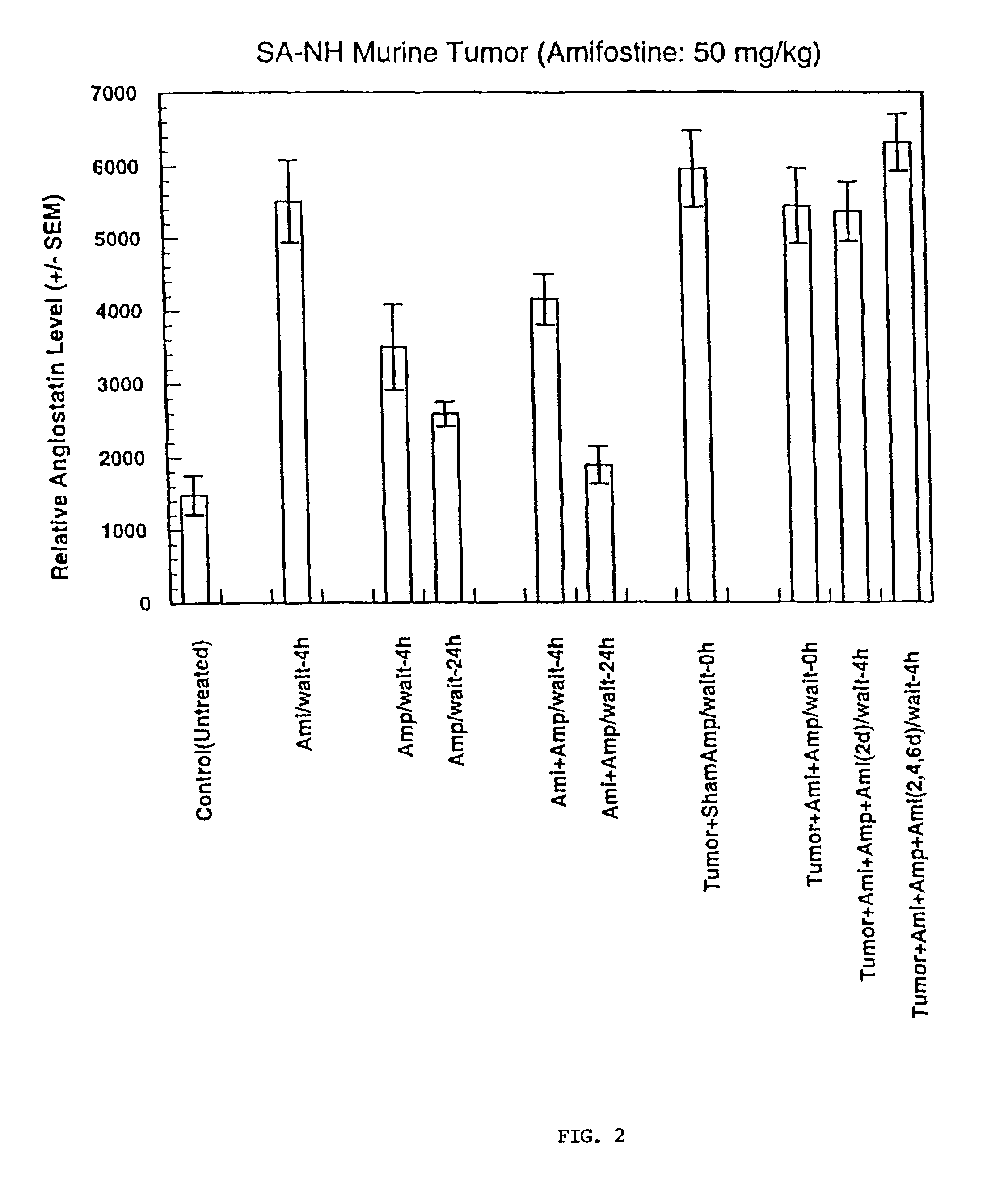
Method for protection against tumor metastasis formation
Methods and pharmaceuticals for inhibiting or preventing metastasis formation in animals, including humans, having primary tumors, through the administration of phosphorothioates including their thiol and disulfide metabolites are disclosed. These compounds stimulate angiostatin levels, inhibit matrix metalloproteinases (MMPs), and stimulate manganese superoxidase dismutase (MnSOD). Phosphorothioates, of which amifostine is an example, can be administered as a combination therapy with traditional cancer therapies, including chemotherapy, radiotherapy, surgery, immunotherapy, hormone therapy and gene-therapy. Inhibition or prevention of metastasis by phosphorothioates is independent of tumor type, including adenocarcinomas and sarcomas.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Nitrogen-containing aromatic heterocyclic compound
Provided is a compound useful as a prophylactic and / or therapeutic agent for bladder cancer.As a result of studies on compounds having FGFR inhibitory action, the present inventors have found that the nitrogen-containing aromatic heterocyclic compounds of the present invention have inhibitory action on FGFR1, FGFR2, and / or FGFR3, particularly, mutant FGFR3, and thus, the present invention has been accomplished. The nitrogen-containing aromatic heterocyclic compound of the present invention can be used as a therapeutic agent for various cancers related to FGFR1, FGFR2, and / or FGFR3, such as lung cancer and hormone therapy-resistant breast cancer, stomach cancer, triple negative breast cancer, endometrial cancer, bladder cancer, and glioblastoma, particularly as a prophylactic and / or therapeutic agent for mutant FGFR3-positive bladder cancer.
Owner:ASTELLAS PHARMA INC +1
Chinese medicine preparation for treating importence
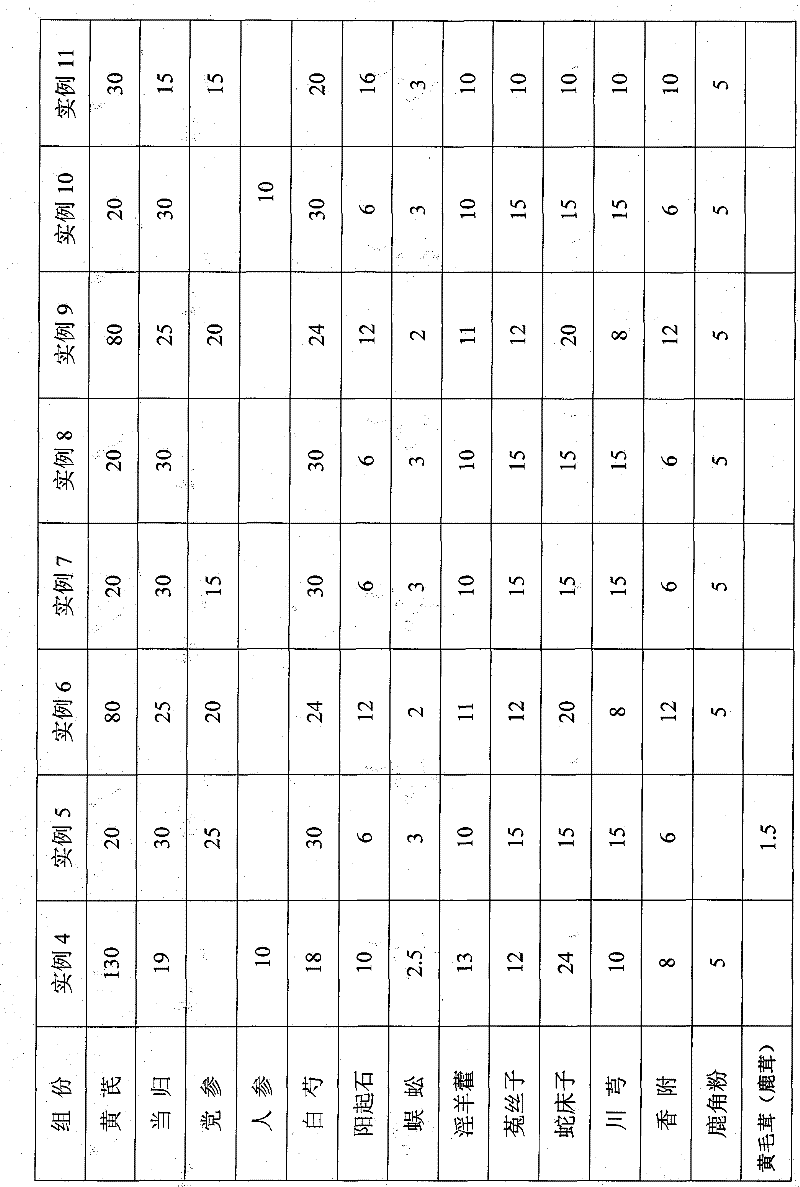
InactiveCN101584790AOvercome incapacityNo side effectsHeavy metal active ingredientsAnthropod material medical ingredientsDiseaseAdemetionine
The present invention provides a traditional Chinese medicine preparation for treating importence, which is the prepared slice comprising the following medicinal materials: 20-180 parts by weight of Astragalus root; 15-30 parts by weight of Chinese angelica; 15-30 parts by weight of pilose asiabell root or 15-20 parts by weight of ginseng; 15-30 parts by weight of white peony root; 6-15 parts by weight of actynolin; 2-3 parts by weight of centipede; 10-15 parts by weight of shorthorned epimedium herb; 10-15 parts by weight of dodder seed; 15-30 parts by weight of common cnidium fruit; 6-15 parts by weight of chuanxiong rhizome; 6-15 parts by weight of nutgrass galingale rhizome; 3-5 parts by weight of powdered antler or 0.5-1.5 parts by weight of hairy antler; and 6-10 parts by weight of liquorice. The Chinese medicine preparation for treating importenance according to the invention applies the method of general nourishment on the basis of the theory of traditional Chinese medicine for regulating the dysfunction of meridian of human organ and the unsmooth running of qi-blood and body fluid. The invention overcomes the defect of unremarkable effect when the method of temperature compensation is singly applied for treating the asynodia according to the traditional Chinese medicine. Simultaneously the side effect caused by singly adopting vasodilation or hormone therapy according to the western medicine. The traditional Chinese medicine of the invention has the characteristics of quick curative effect, no toxic or side effect, flexible theory and radical treatment. Simultaneously according to the principle of treating the root of the disease in the theory of traditional Chinese medicine, the medicine is added or reduced according to the illness state on the basis of general nourishment for adopting the symptoms of different patients or the illness states of a same patient in different periods.
Owner:李进才
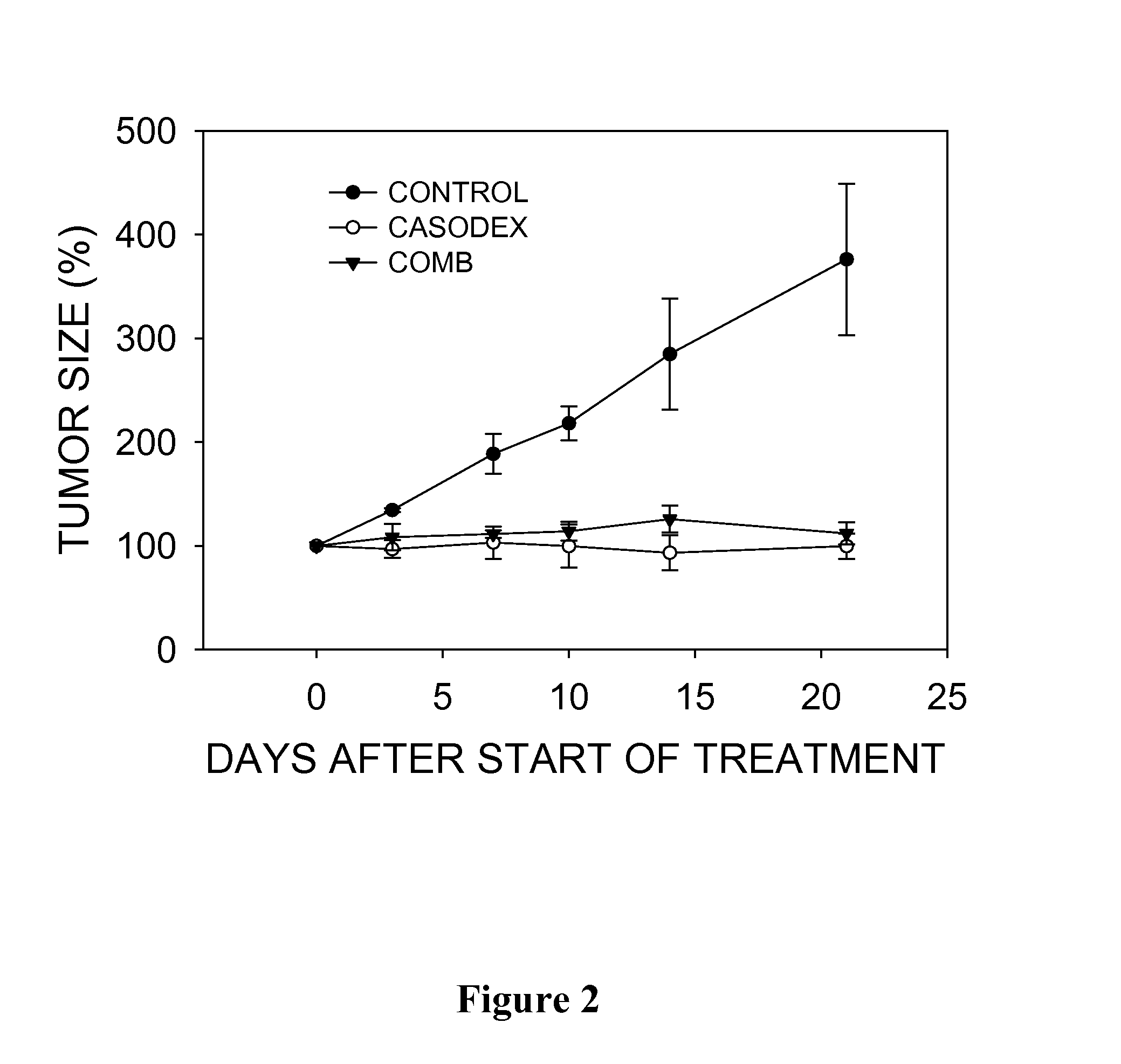
Novel treatment of prostate carcinoma
ActiveUS20130219528A1Inhibiting and delaying growthReduce the amount requiredBiocidePeptide/protein ingredientsMedicineProstate cancer
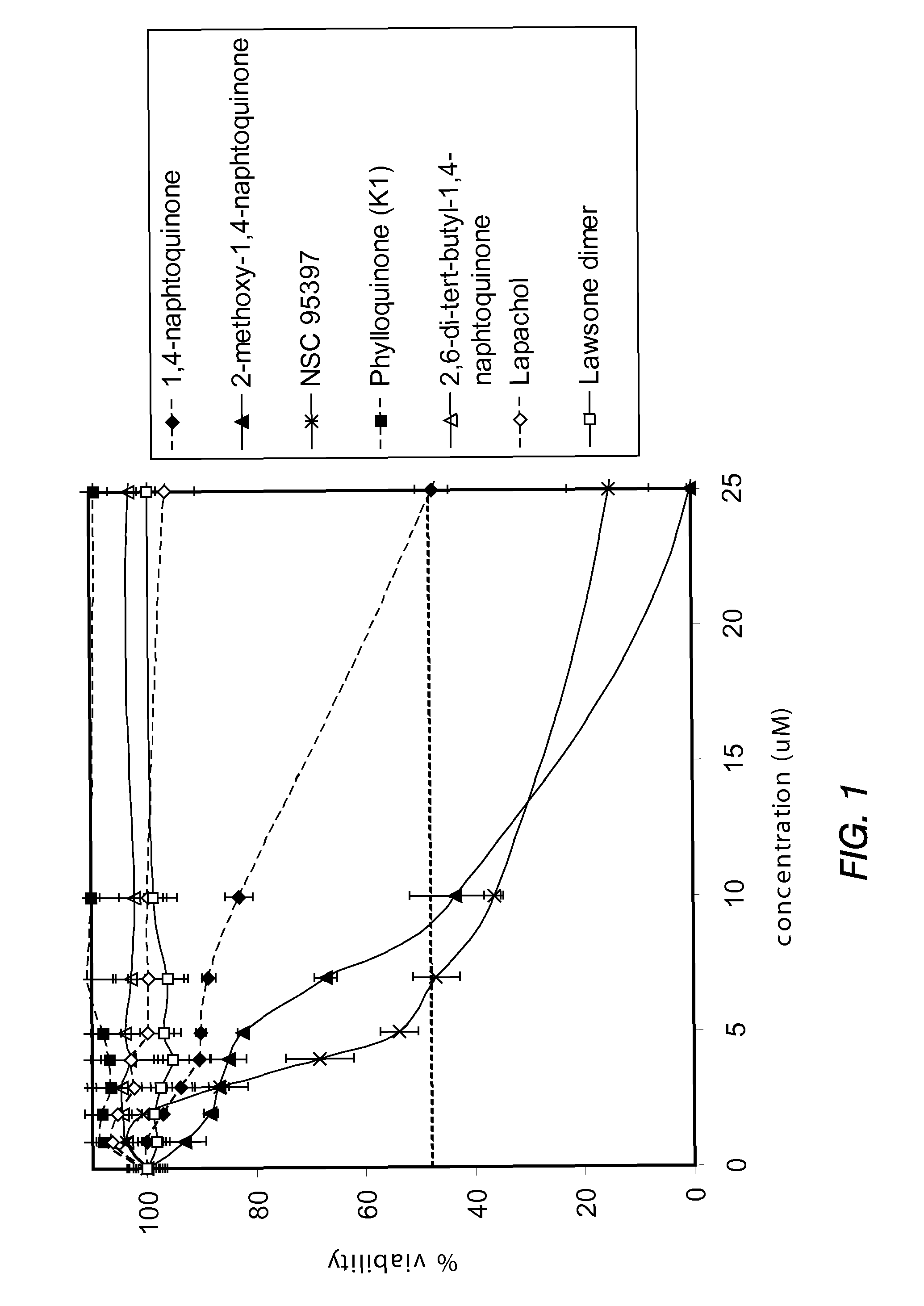
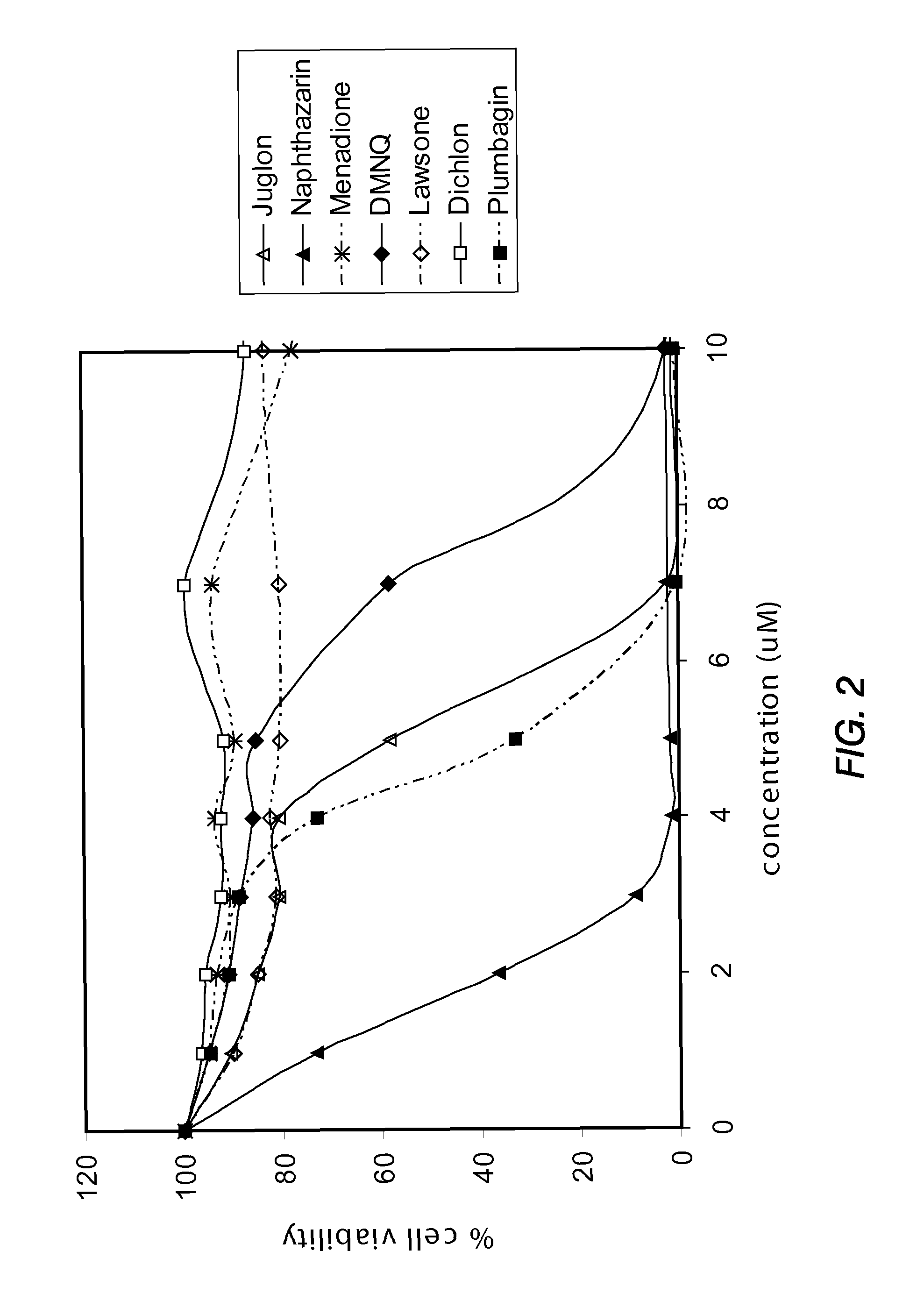
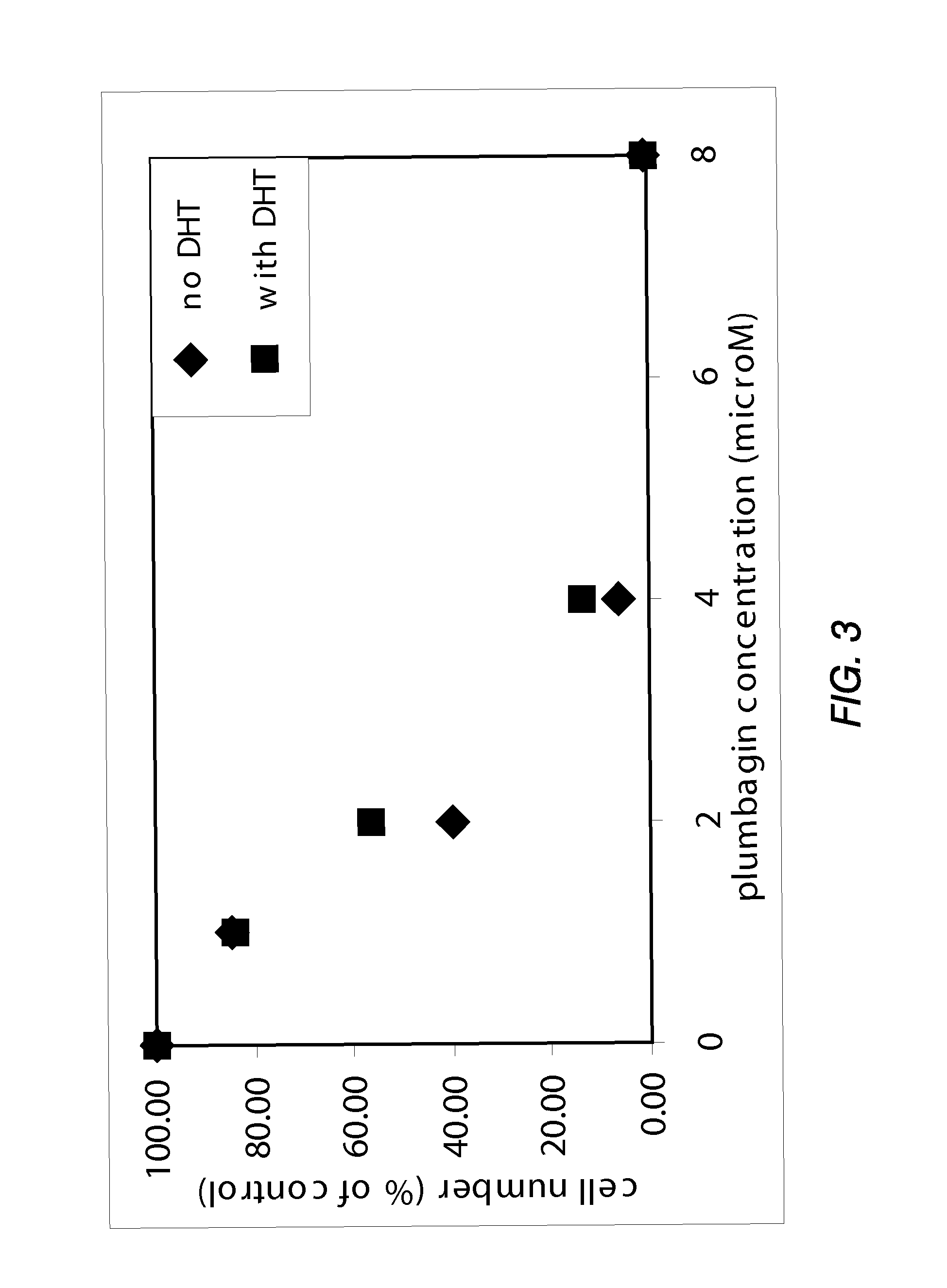
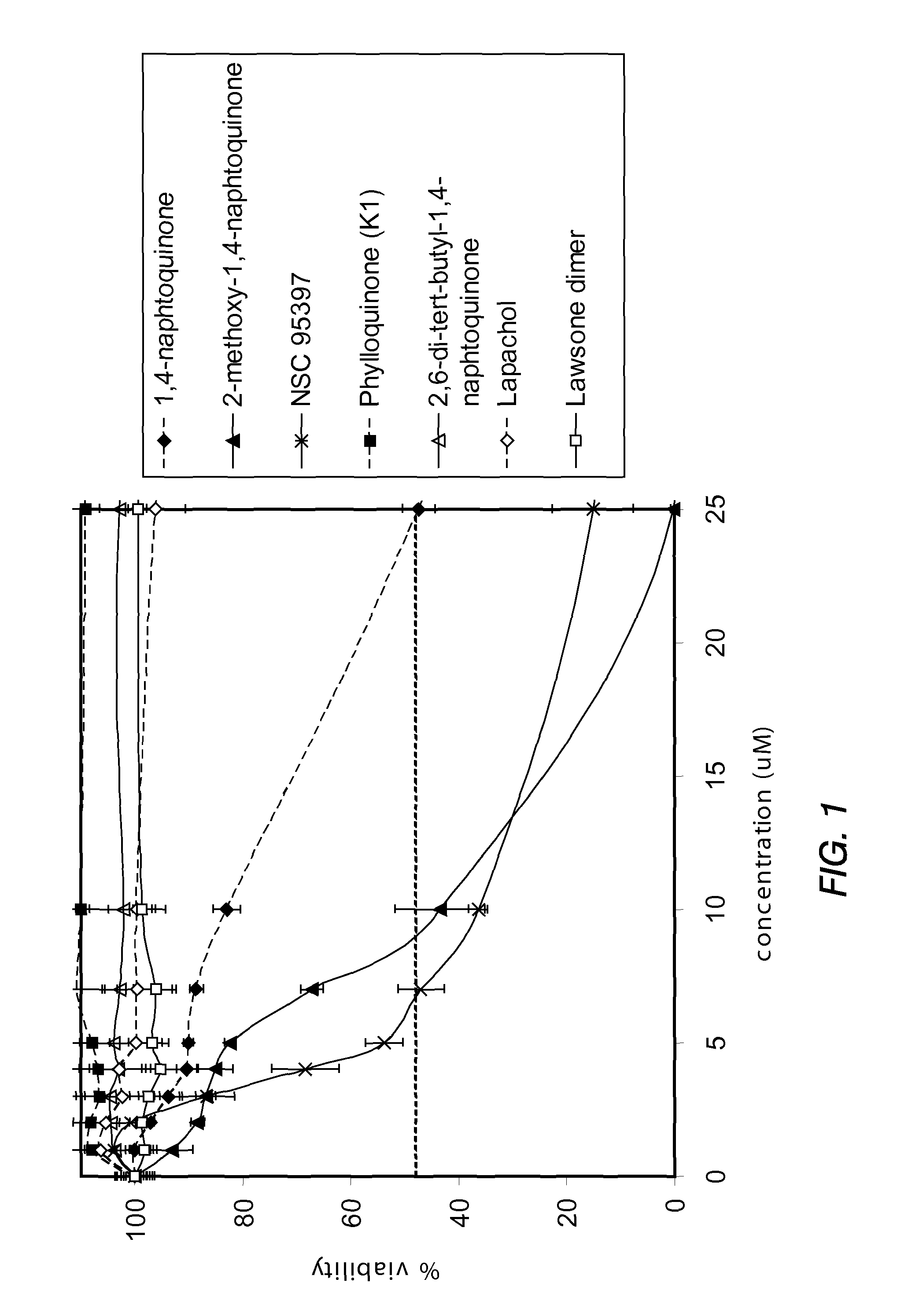
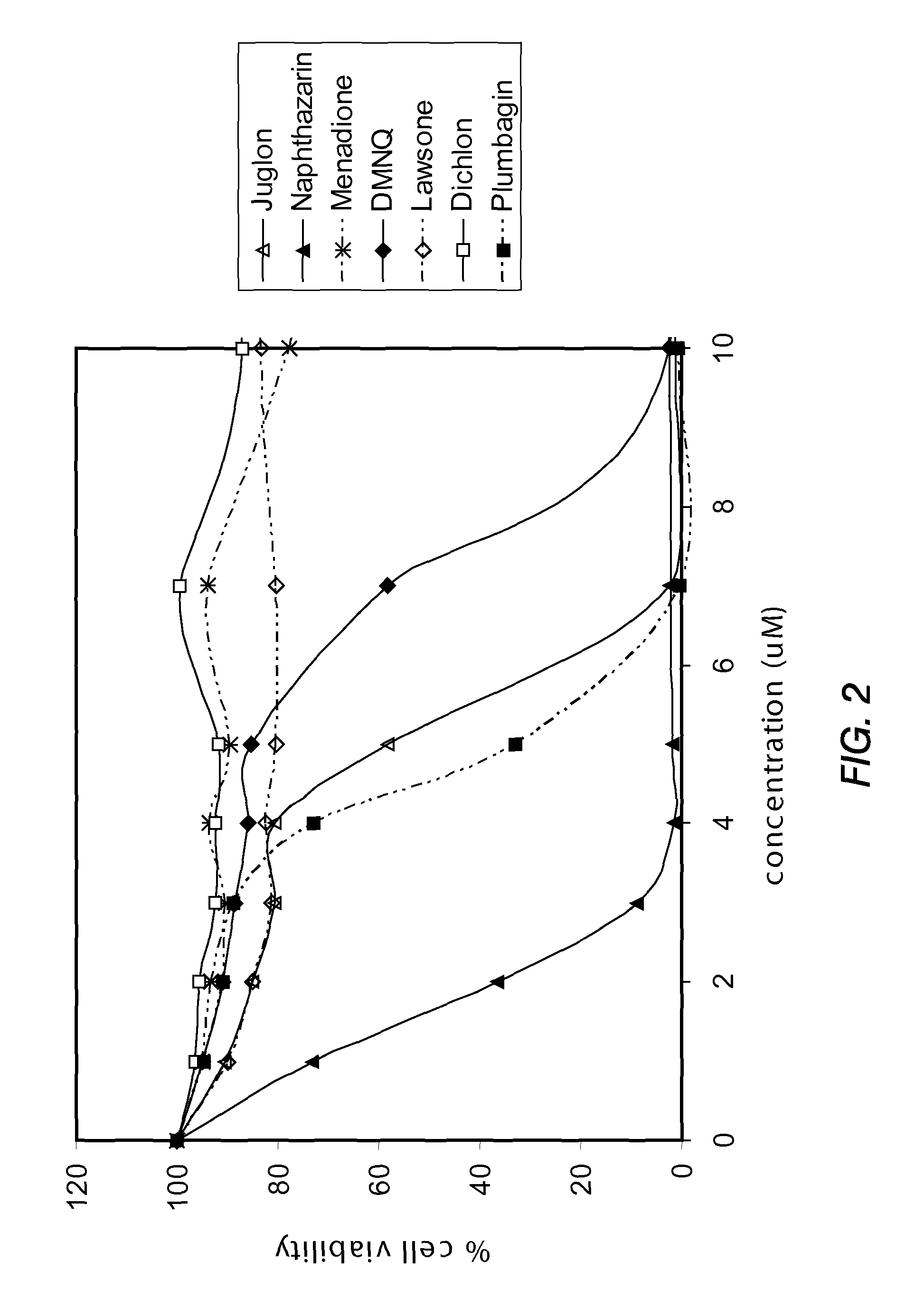
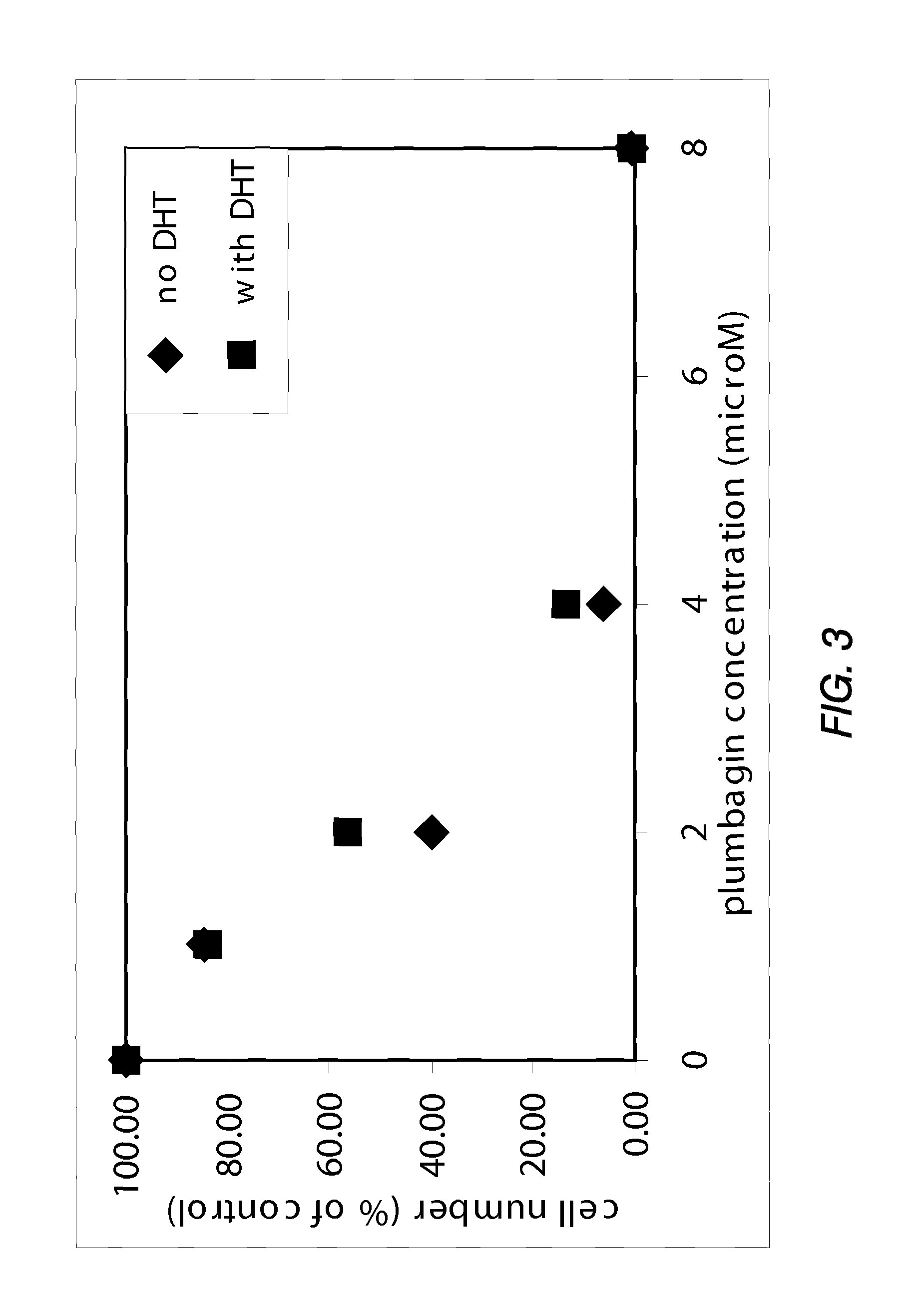
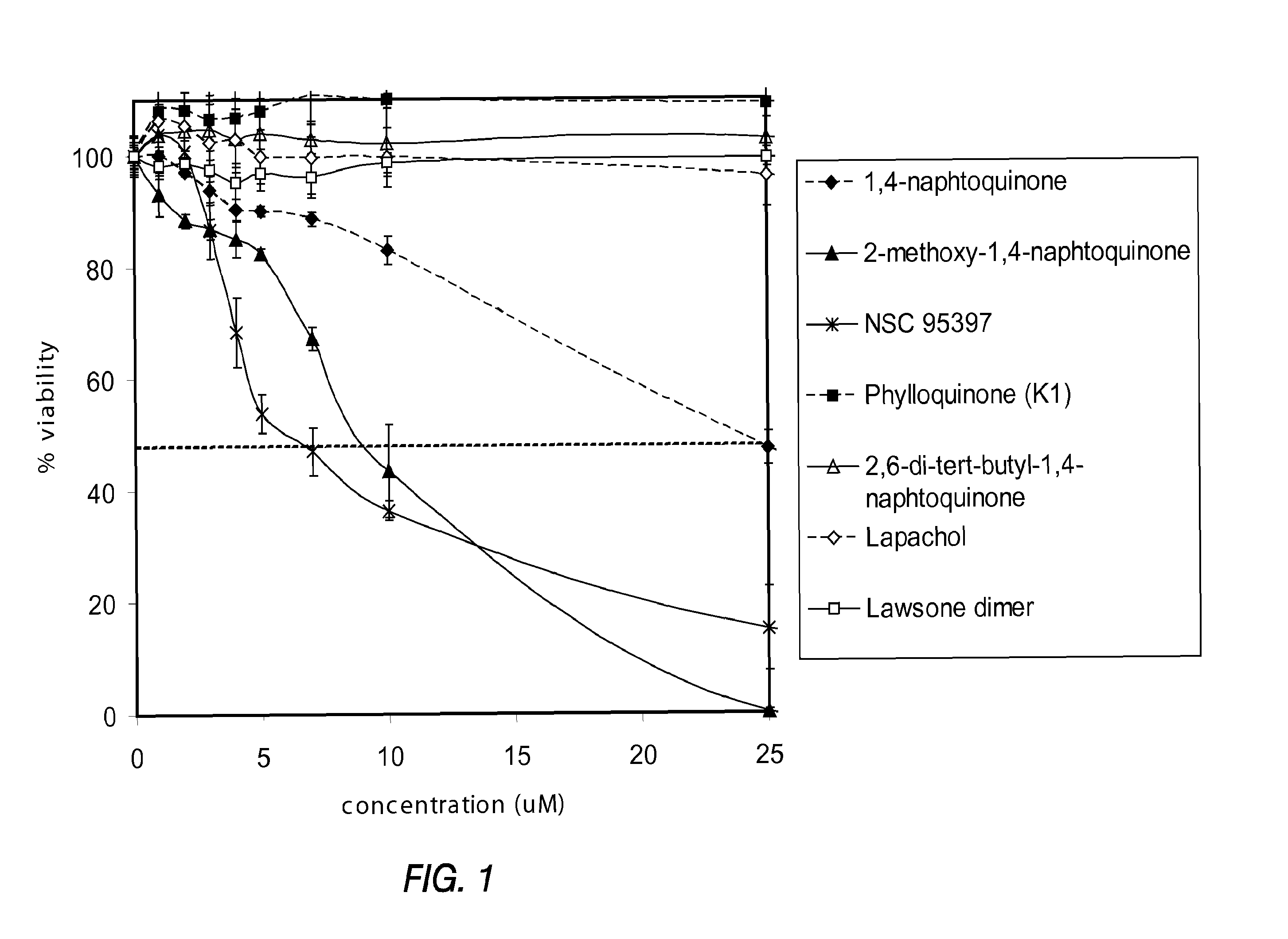
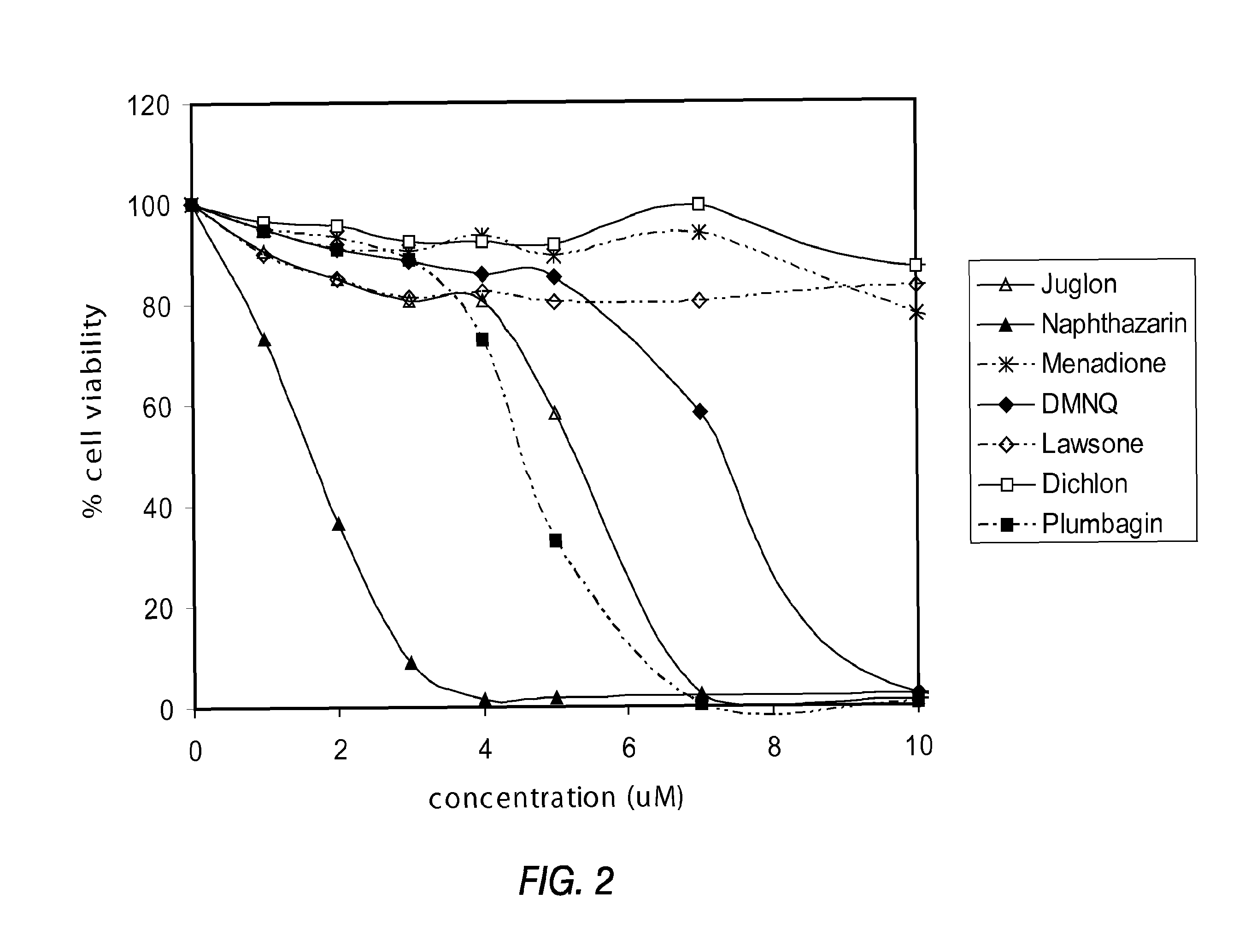
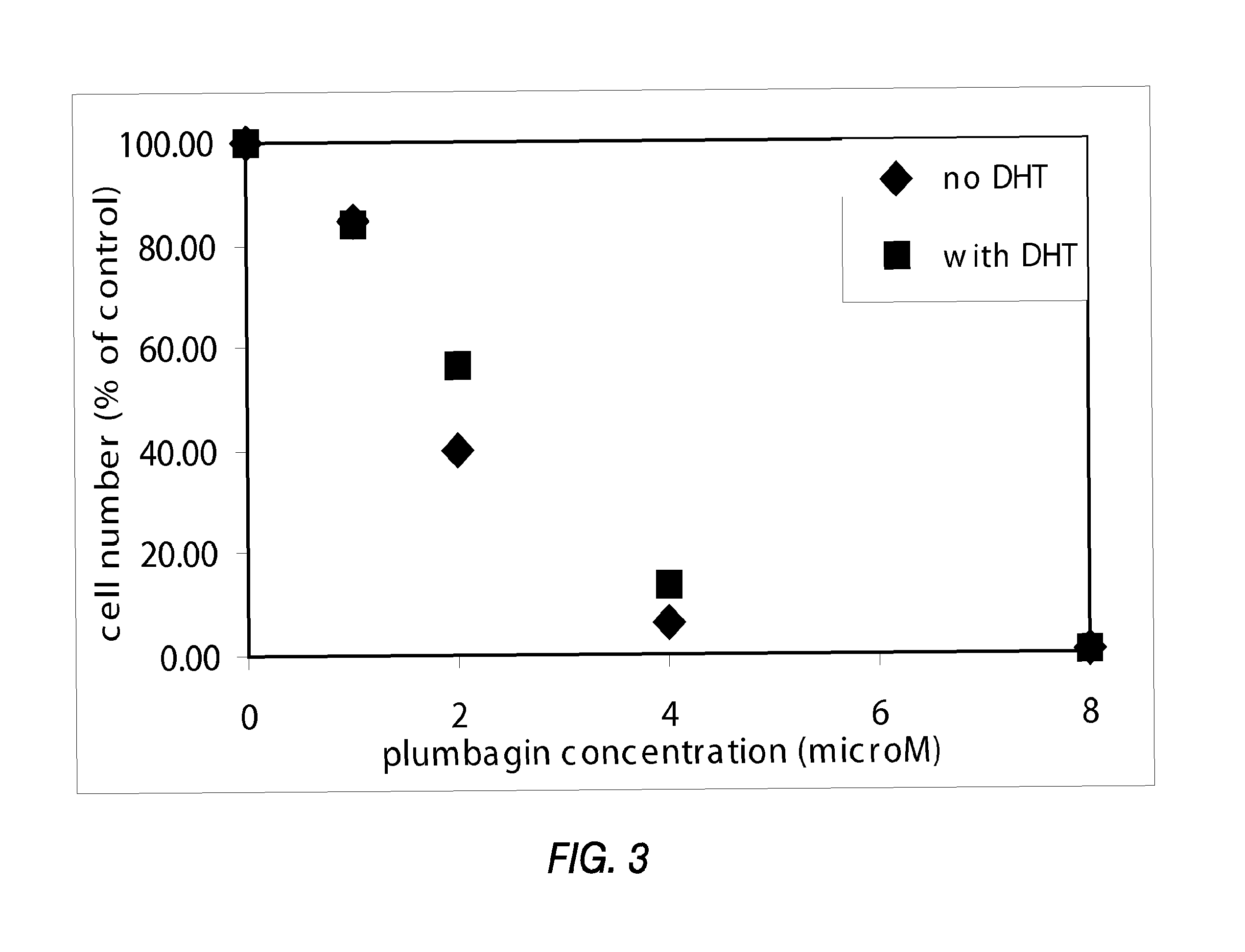
Disclosed herein are naphthoquinone analogs, such as plumbagin, pharmaceutical compositions that include naphthoquinone analogs, such as plumbagin, and methods of treating diseases and / or conditions such as cancer with naphthoquinone analogs, such as plumbagin. Also included are combination therapies wherein a naphthoquinone analog, such as plumbagin, and a hormone therapy agent are provided to a subject suffering from a condition such as cancer.
Owner:PELLFICURE PHARMA
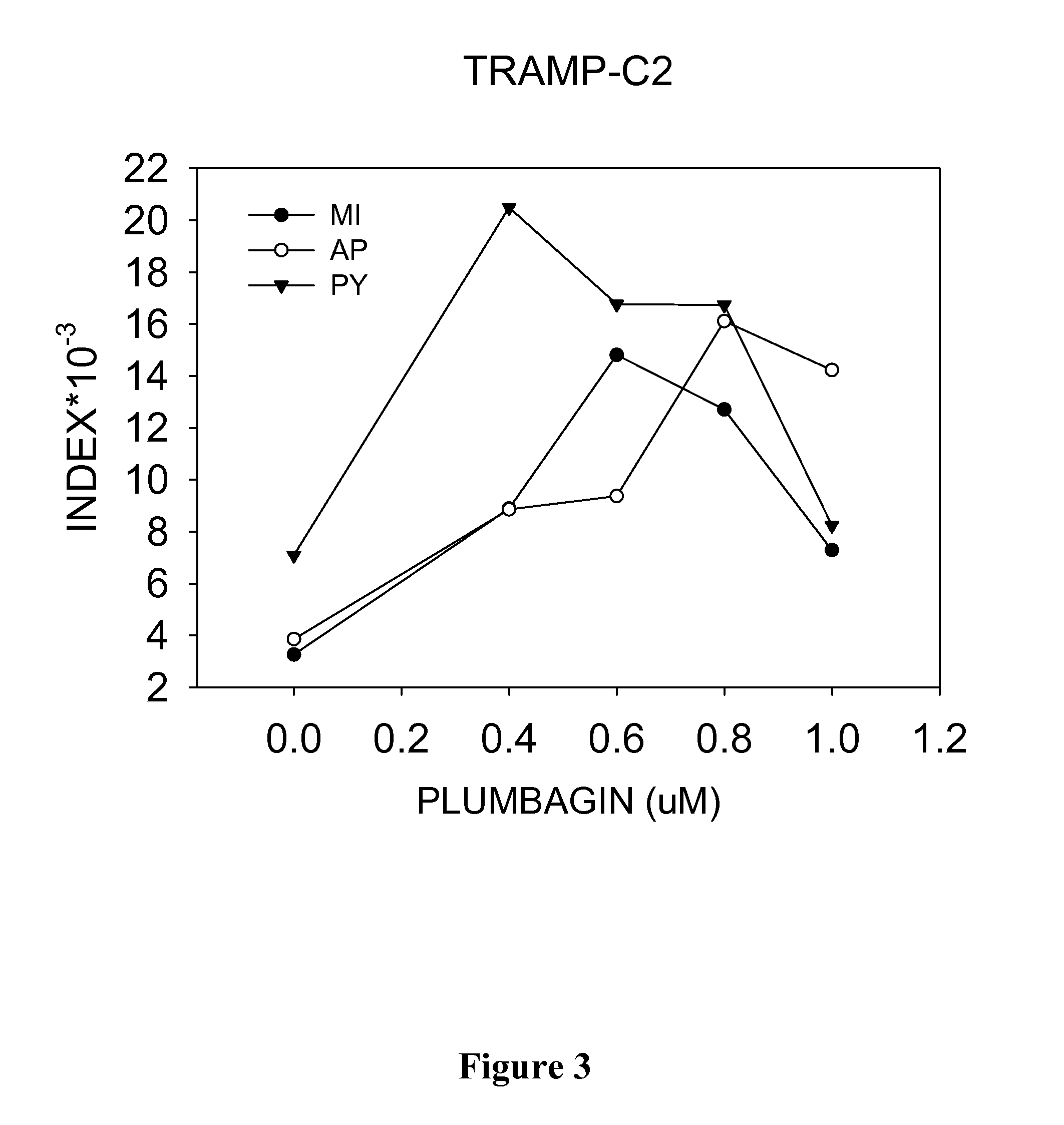
Novel therapy for prostate carcinoma
InactiveUS20160022606A1Growth inhibitionInhibits and delays onsetBiocideAnimal repellantsLyase activityHormone dependence
Disclosed herein are methods of inhibiting or delaying the growth of androgen-dependent prostate cancer, and / or inhibiting or delaying the onset of castration-resistant prostate cancer (CRPC) by administering naphthoquinone analogs, such as plumbagin, and specified hormone therapy agents, including selective inhibitors of 17,20-lyase activity of CYP 17.
Owner:PELLFICURE PHARMA
Composition for prostate health
InactiveUS8221803B1Increase blockingReducing elevated PSA levelBiocideHydrocarbon active ingredientsDiseaseSaw palmetto extract
The present invention provides an effective, all-natural, non-toxic, non-hormonal composition consisting of vitamin D3, vitamin E, selenium, green tea extract, saw palmetto berry extract, isoflavanoids, and lycopene for prostate health. The invention provides compositions and methods to prevent, alleviate, and / or treat symptoms associated with prostate conditions and diseases. The prostate health composition may be used to supplement medical treatment such as radiation therapy, chemotherapy, and hormone therapy.
Owner:ONCONATURAL SOLUTIONS
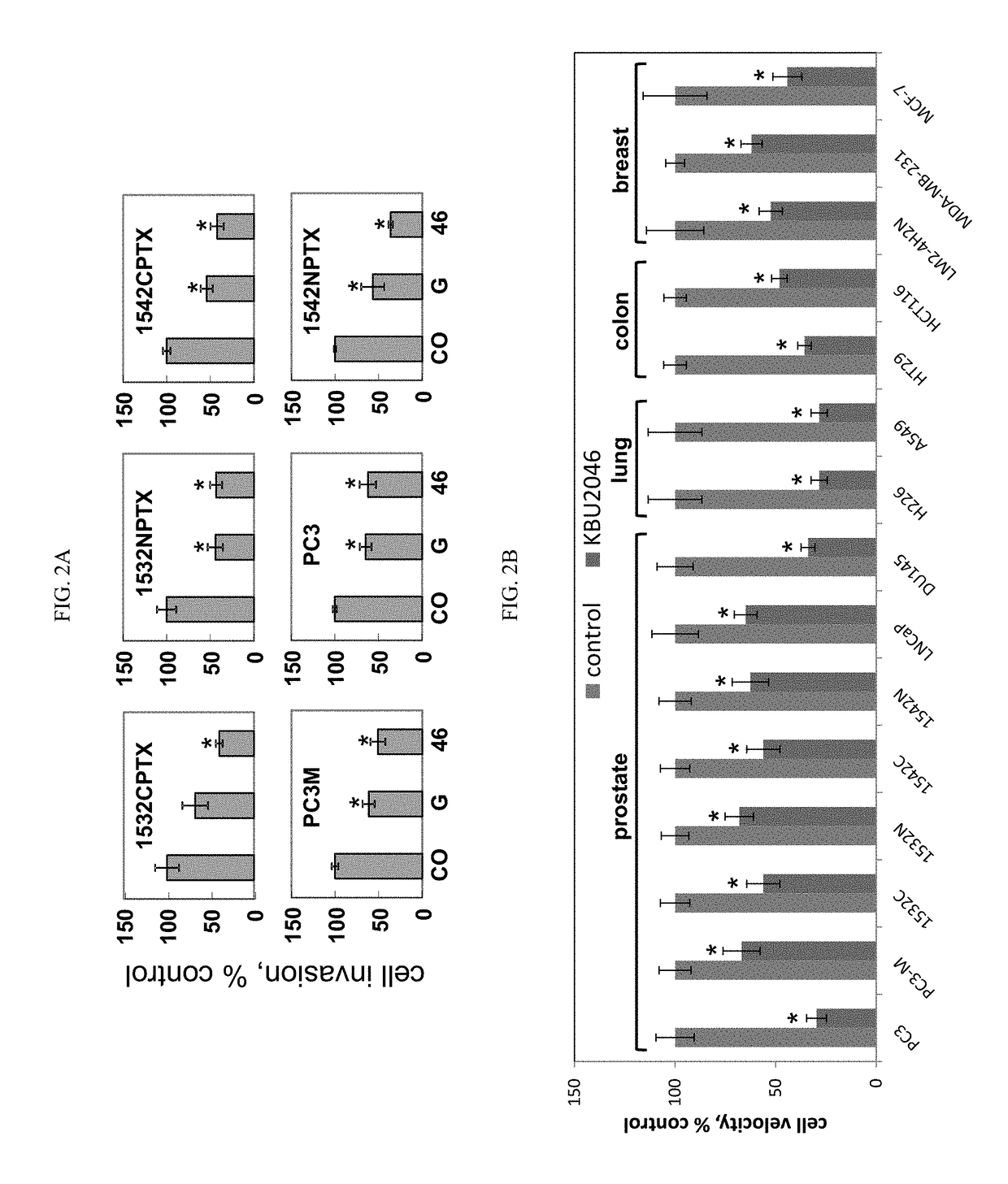
Inhibition of cancer cell motility
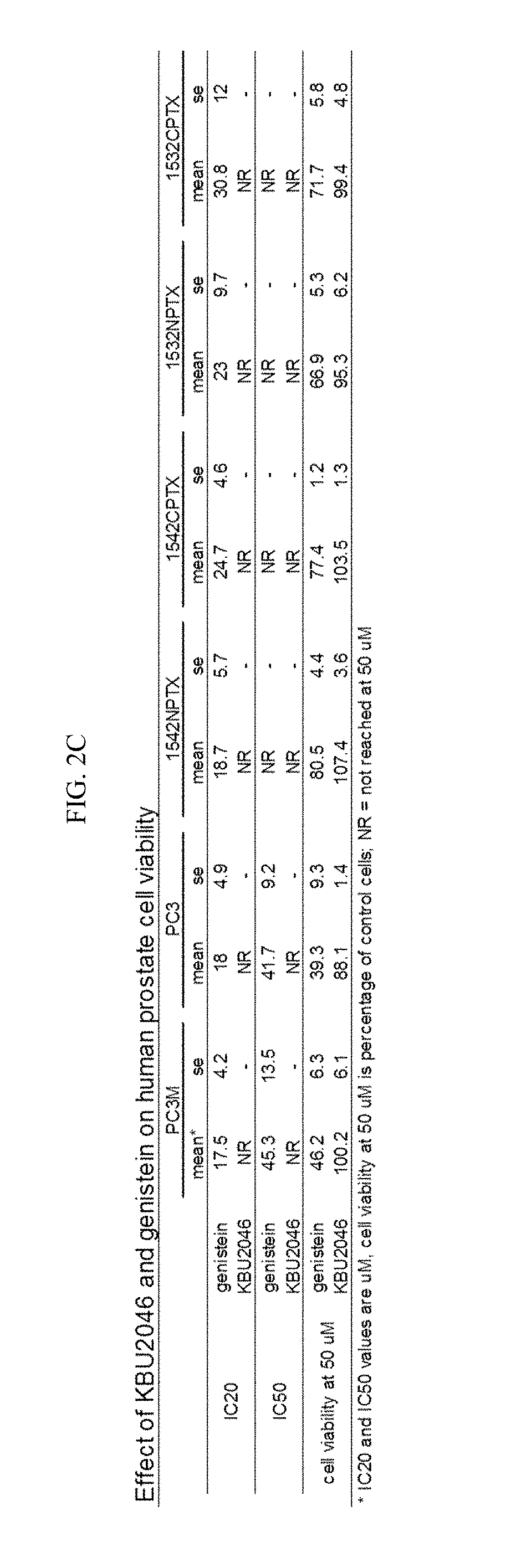
ActiveUS10231949B2Improve efficiencyOvercome hormone resistanceOrganic active ingredientsDigestive systemCancer cellLymphatic Spread
Provided herein are compositions and methods for inhibiting cancer cell motility and / or metastasis. In particular embodiments, KBU2046 (or an analog thereof) and one or more additional therapies (e.g., cancer therapies (e.g., hormone therapies and chemotherapies) are provided to inhibit cancer cell motility, inhibit metastasis, and / or treat cancer (e.g., prostate cancer, lung cancer, breast cancer, colon cancer, etc.).
Owner:NORTHWESTERN UNIV
Therapy of prostate cancer with CTLA4 antibodies and hormonal therapy
InactiveCN101146552APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHistrelinFlutamide
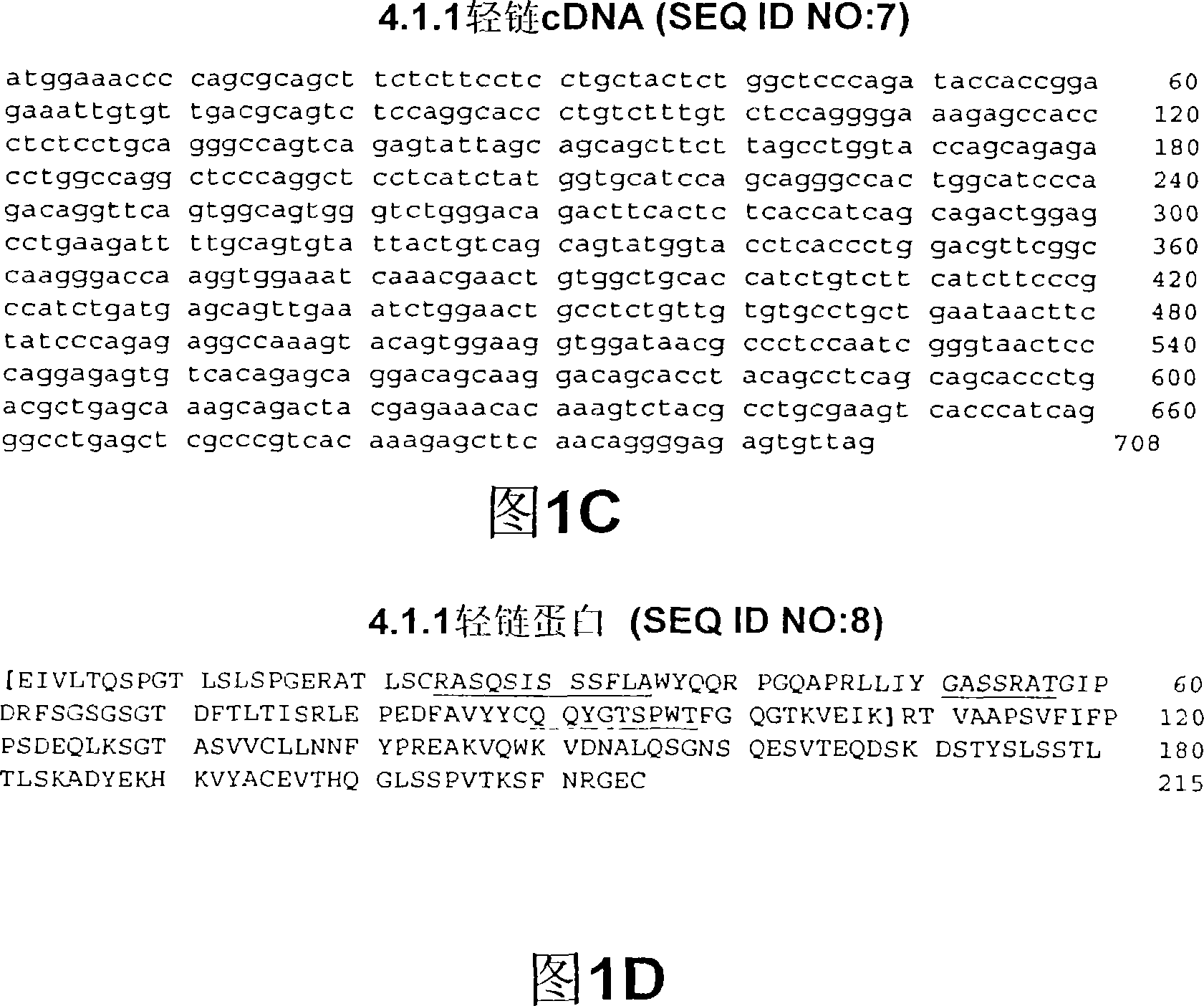
The present invention relates to a method for treating prostate cancer, which comprises administering an anti-CTLA4 antibody, or an antigen-binding portion thereof, especially a human antibody against human CTLA4, such as antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2 , 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-101 and 10D1) , in combination with hormone therapy. Hormone therapy agents include, inter alia, antiandrogens (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), GnRH antagonists (e.g., ababa Rick and histrelin), and LH-RH agonists (eg, leuprolide, goserelin, and buserelin). The present invention relates to neoadjuvant therapy, adjuvant therapy, treatment of elevated PSA, first-line treatment, second-line treatment and third-line treatment of localized or metastatic prostate cancer.
Owner:PFIZER PROD INC
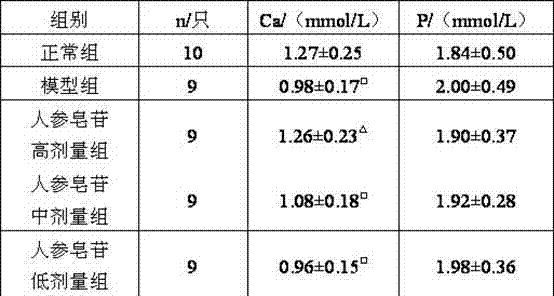
A kind of application of ginsenoside
InactiveCN102283877AExpand the scope of medicinal useReduce osteoporosisSkeletal disorderPlant ingredientsGlucocorticoidCurative effect
The invention relates to an application of ginsenoside in the preparation of medicine for treating osteoporosis, and the osteoporosis is glucocorticoid-induced osteoporosis. The advantages of the present invention are: the present invention provides a new application of ginsenosides, which broadens the medicinal scope of ginsenosides; At the same time, it can effectively prevent and treat glucocorticoid-induced osteoporosis, and reduce the osteoporosis caused by hormone therapy in patients; Better treatment for patients with steroid-induced osteoporosis.
Owner:SHANGHAI HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Methods and compositions using thalidomide for the treatment and management of cancers and other diseases
Methods of treating, preventing and / or managing cancer and diseases and conditions associated with or characterized by unwanted angiogenesis are disclosed. Particular methods include administration of thalidomide alone or in combination with a second active ingredient. The invention also relates to a method of reducing or avoiding adverse side effects associated with chemotherapy, radiotherapy, hormone therapy, biological therapy or immunotherapy comprising administering thalidomide. Pharmaceutical compositions, single unit dosage forms and kits suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
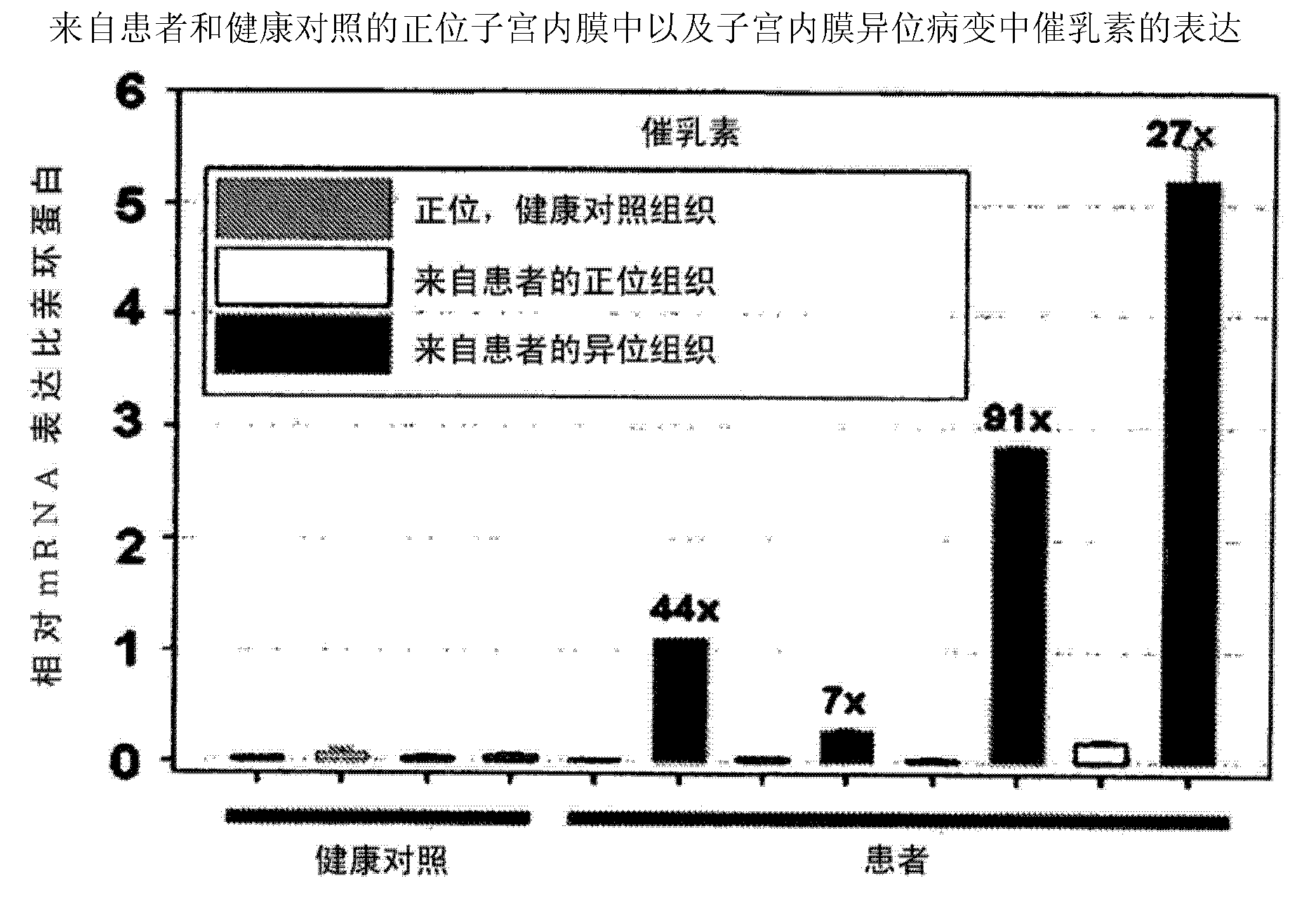
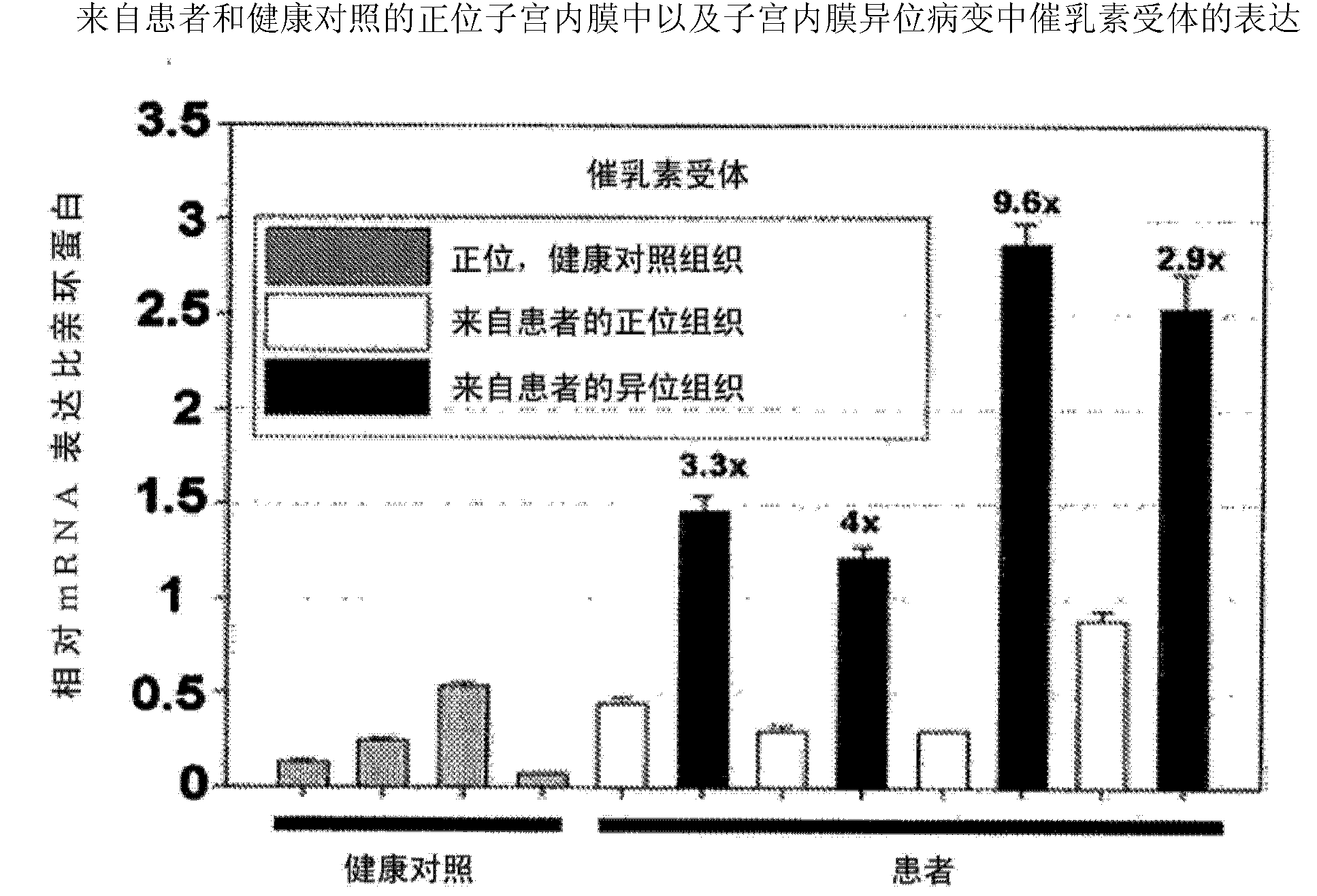
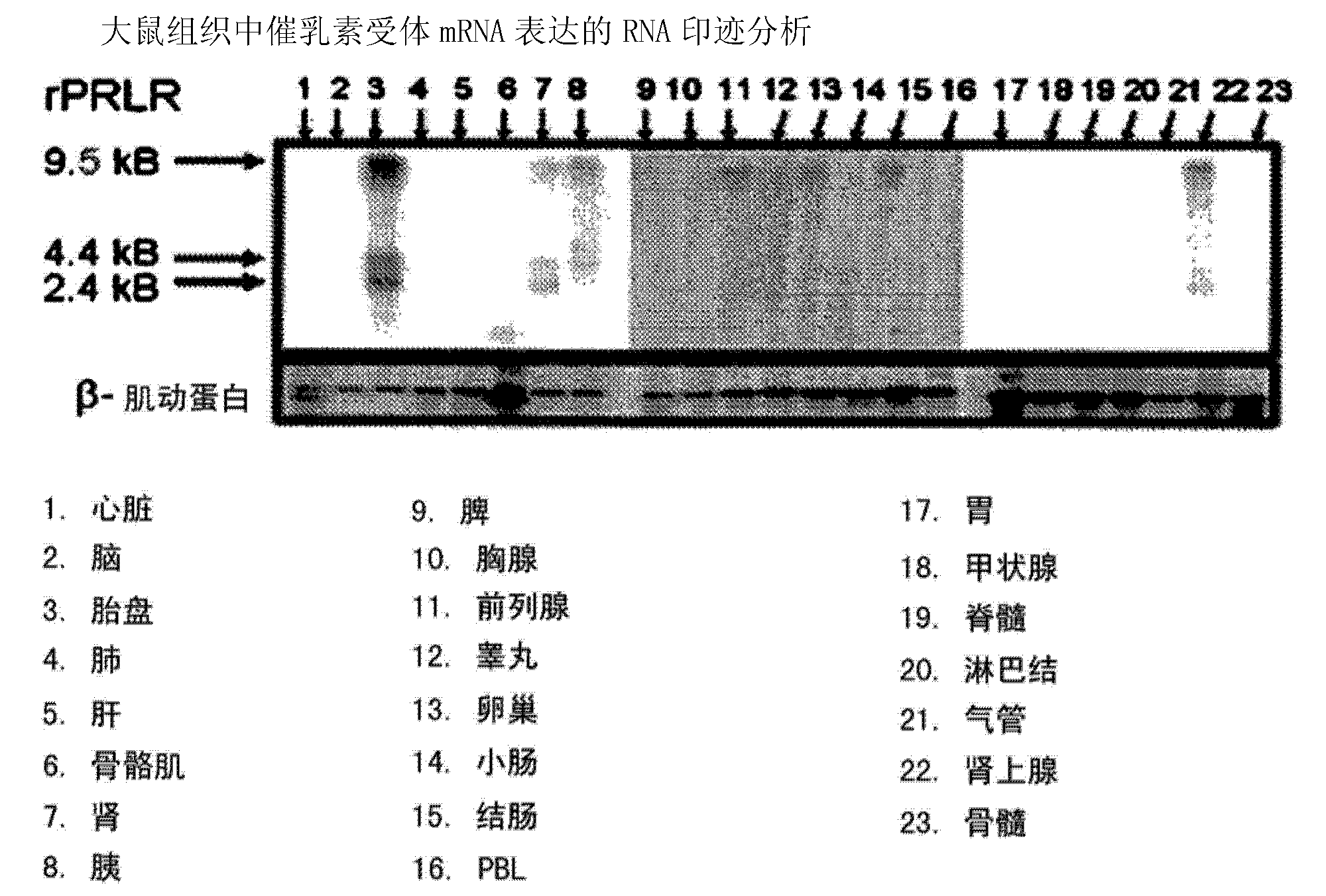
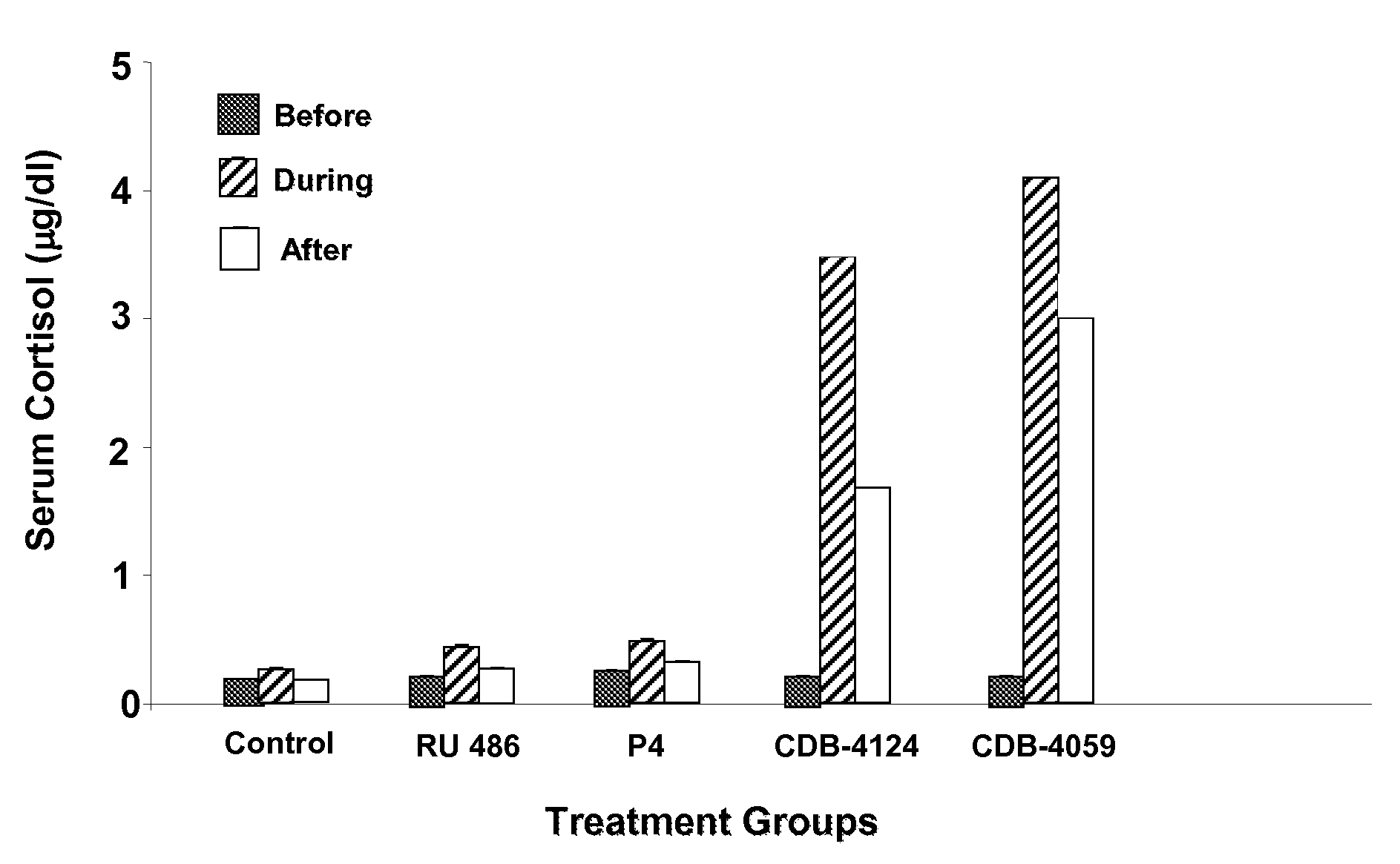
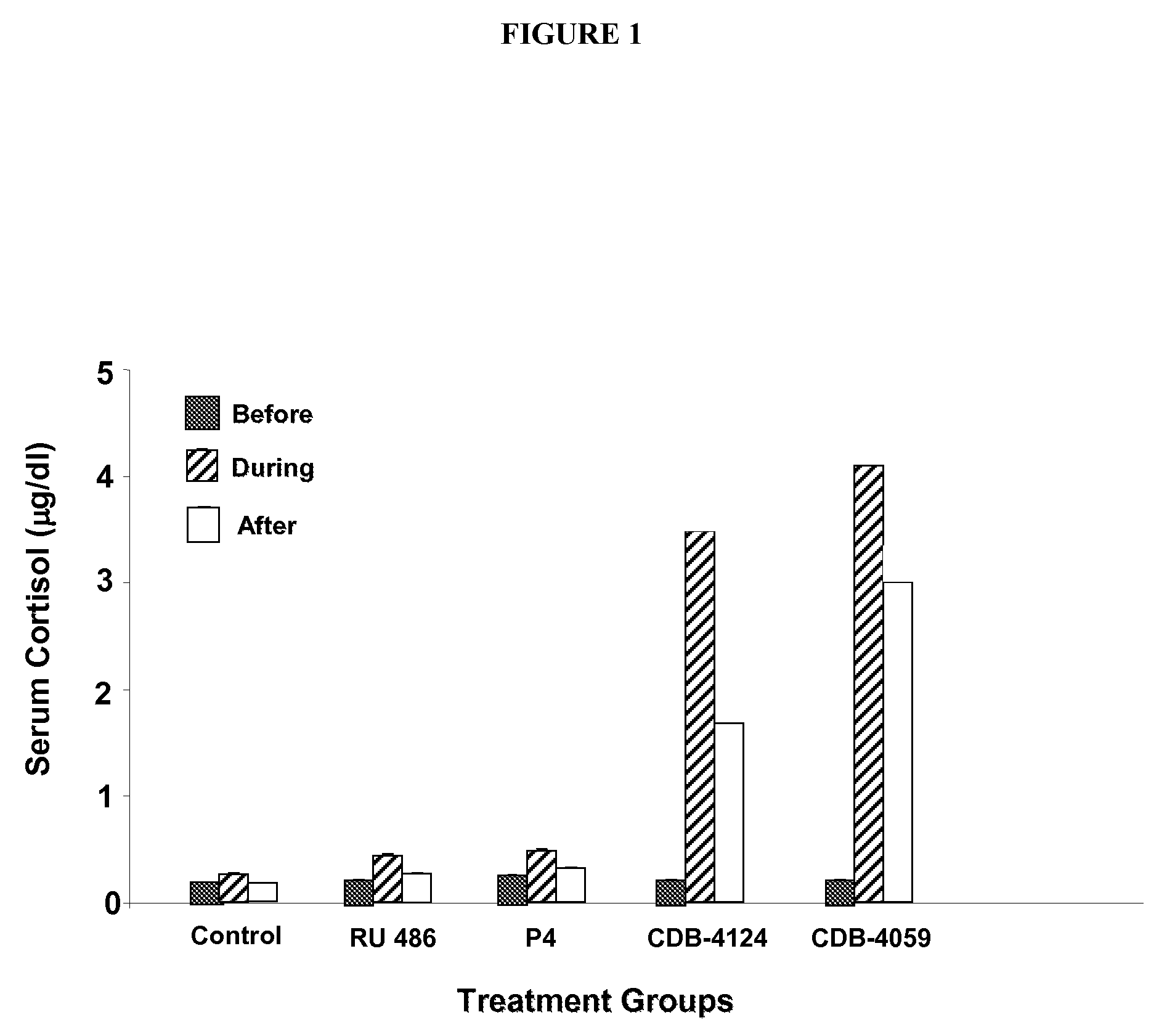
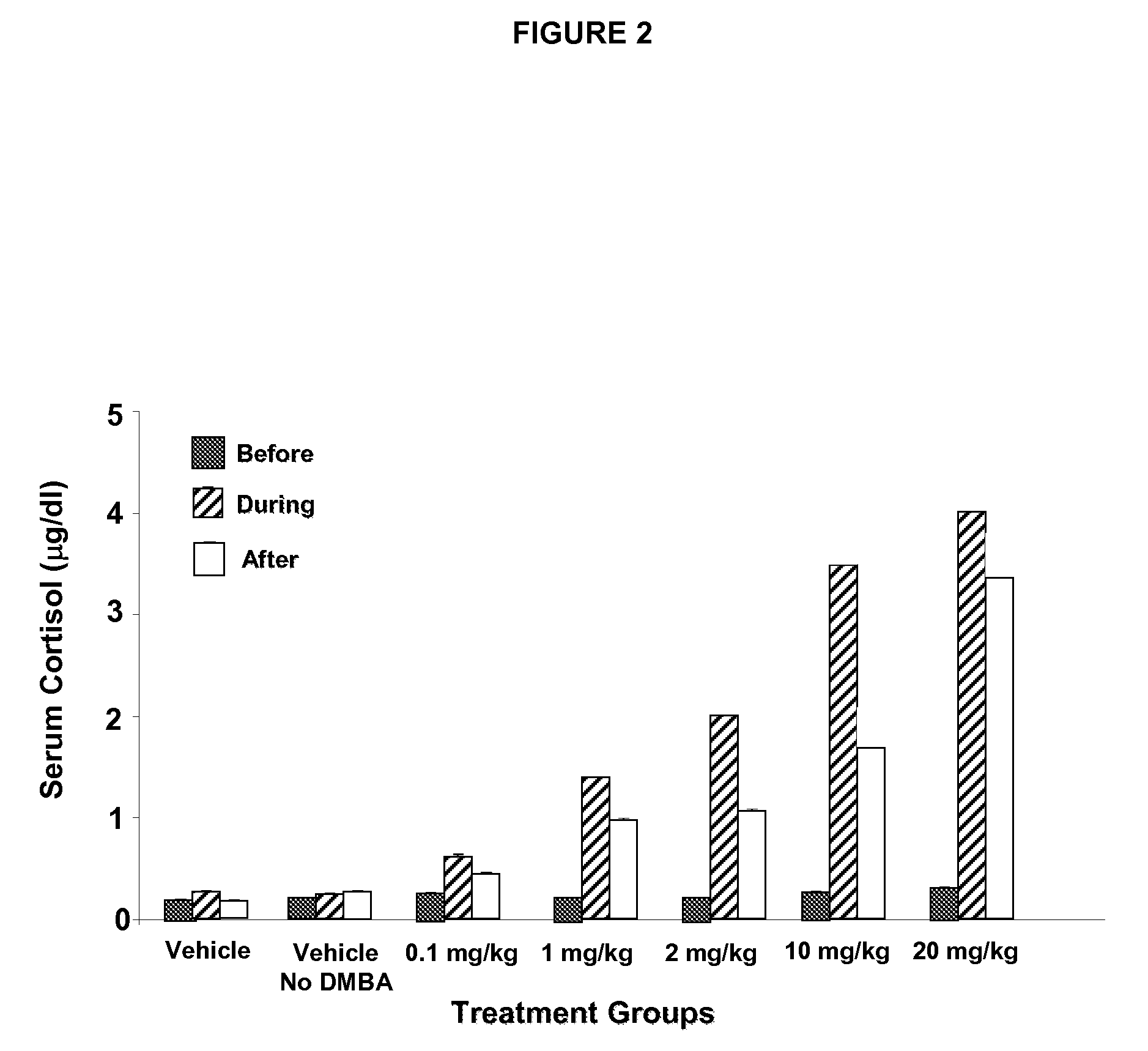
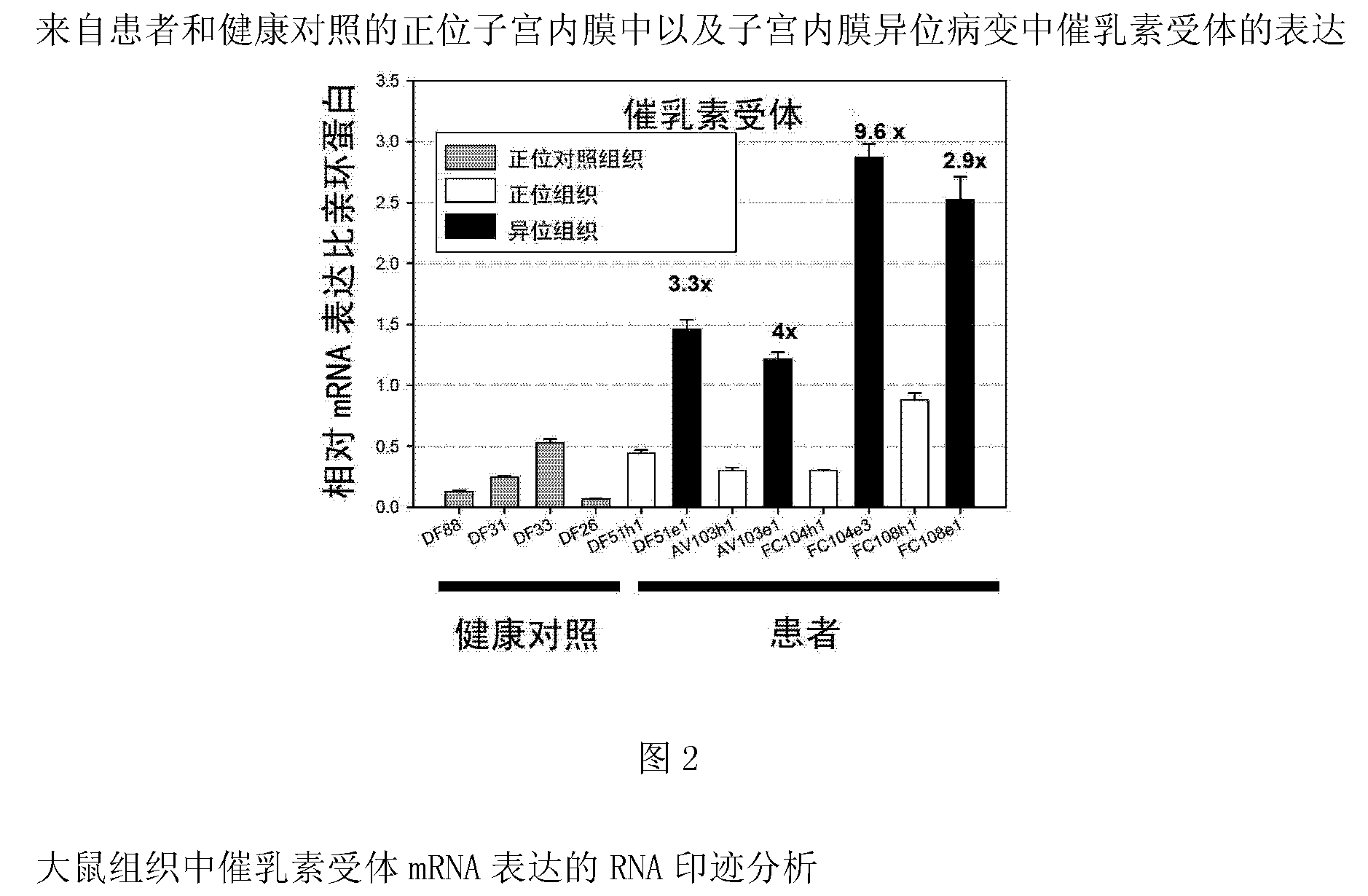
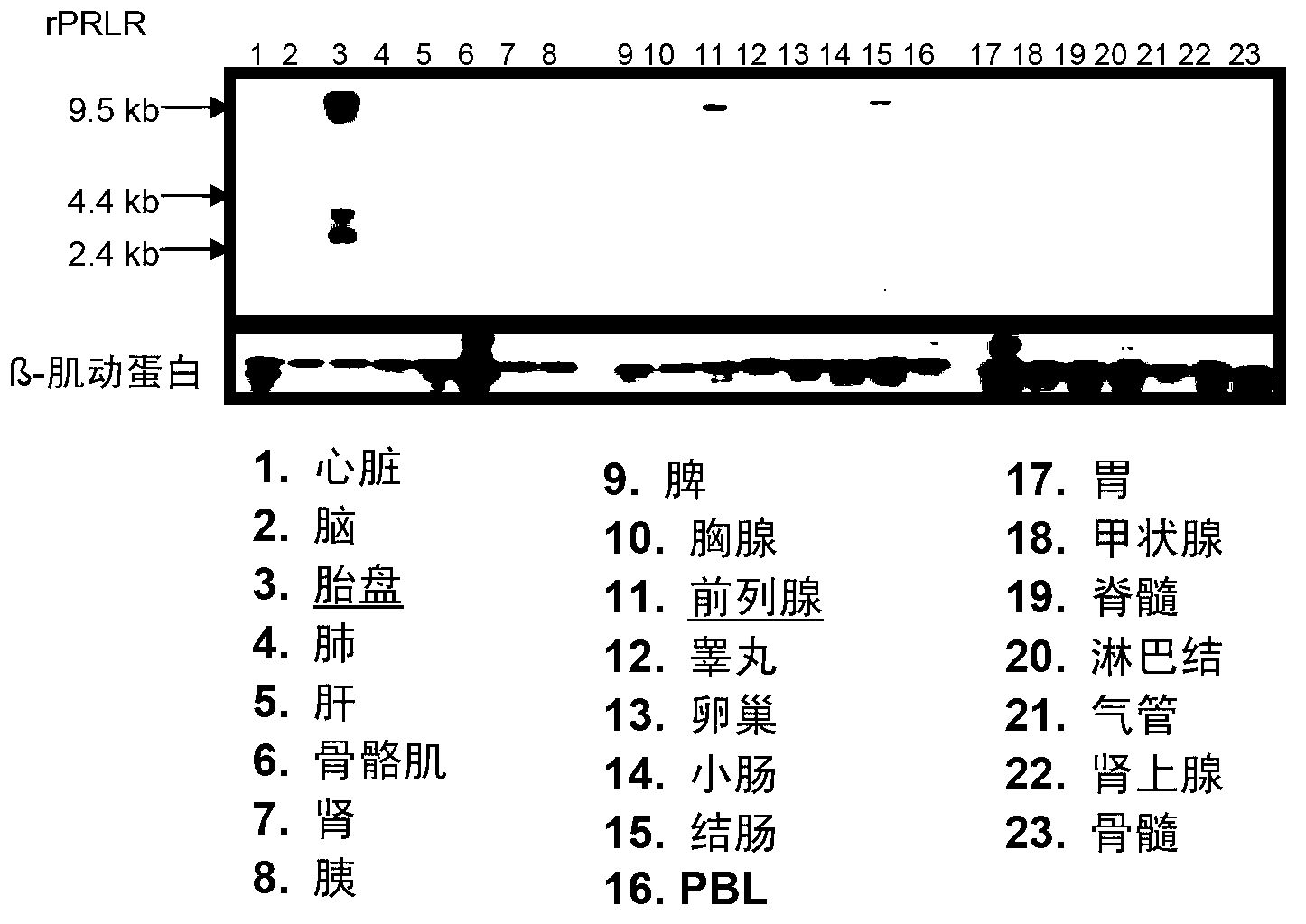
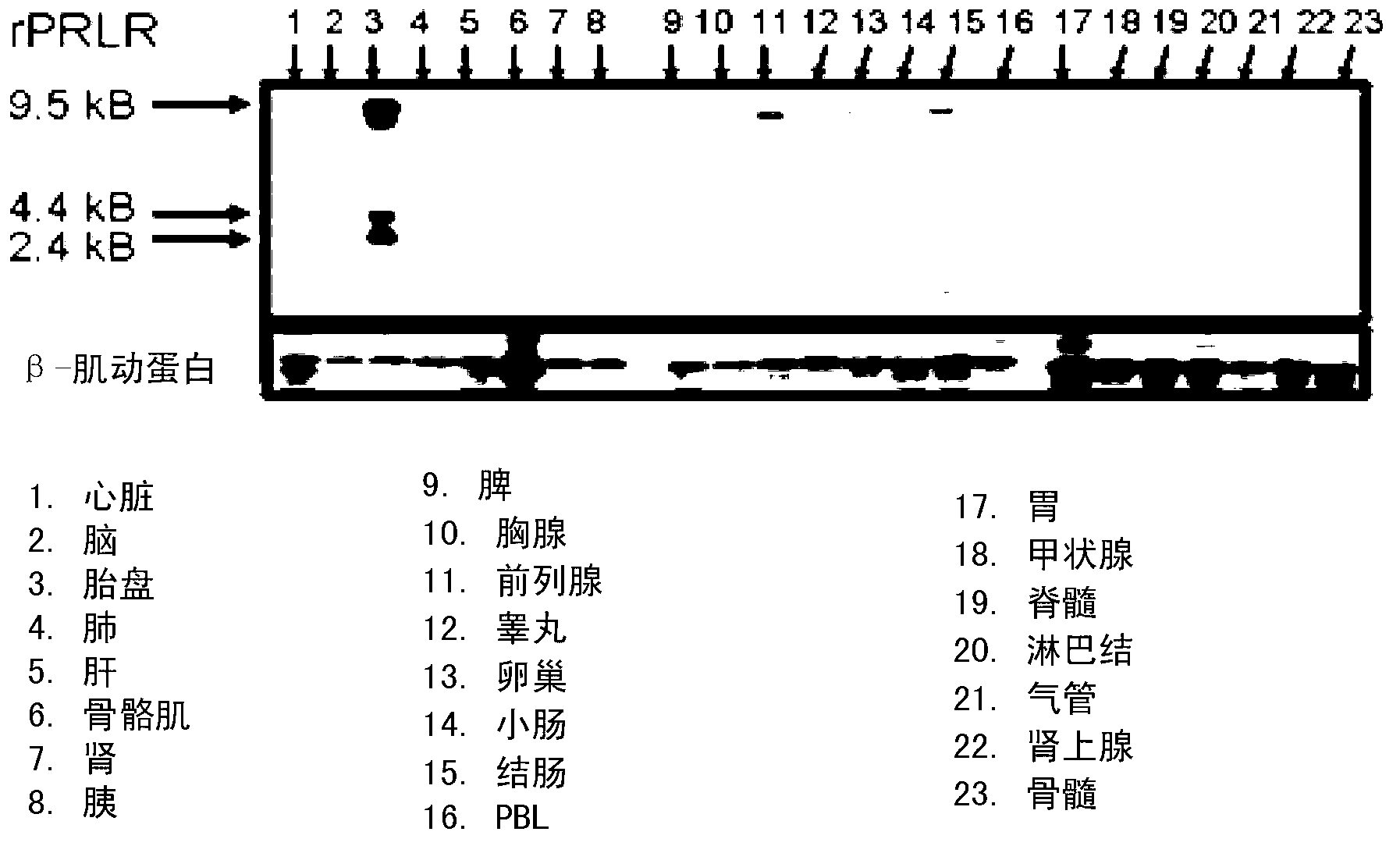
Neutralizing prolactin receptor antibodies and their therapeutic use
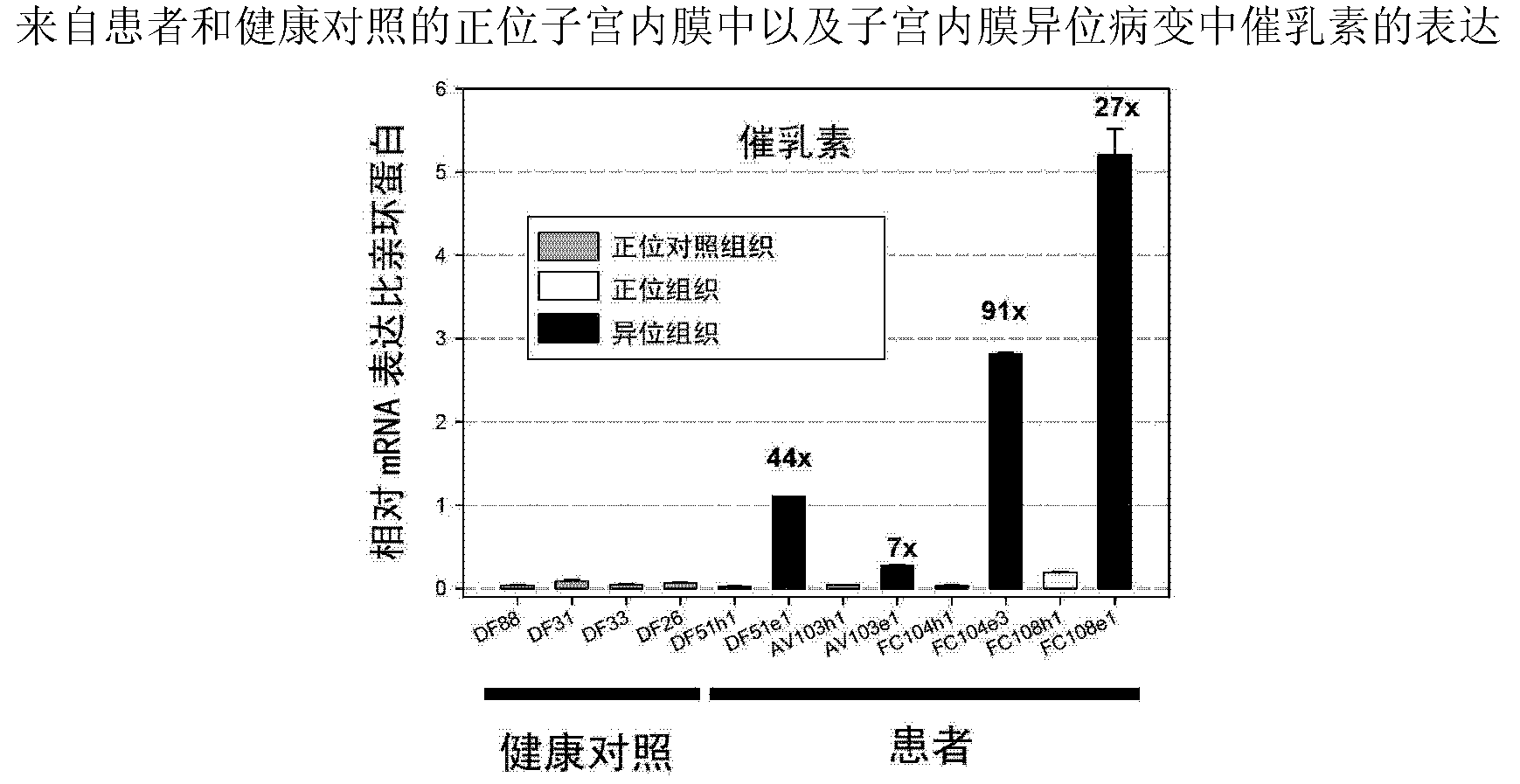
The present invention is directed to neutralizing prolactin receptor antibodies 002-H06 and antigen binding fragments, pharmaceutical compositions containing them and their use in the treatment or prevention of benign disorders and indications mediated by the prolactin receptor such as endometriosis, adenomyosis, non-hormonal female contraception, benign breast disease and mastalgia, lactation inhibition, benign prostate hyperplasia, fibroids, hyper- and normoprolactinemic hair loss, and cotreatment in combined hormone therapy to inhibit mammary epithelial cell proliferation. The antibodies of the invention block prolactin receptor-mediated signaling.
Owner:BAYER IP GMBH
Compositions and methods for treating dysfunctional uterine bleeding
InactiveUS20090118253A1Treating and preventing anemiaPrevent anemiaOrganic active ingredientsSexual disorderSubject matterProgesterones
The subject matter of the instant invention is pertinent to the field of hormone therapy. More specifically, the subject matter of the instant invention concerns methods of treating dysfunctional uterine bleeding. The instant invention is also relevant to the treatment and / or prevention of anemia in patients with dysfunctional uterine bleeding. Compositions for practicing the methods, comprising progesterone antagonists are also disclosed. Embodiments of the instant invention also disclose methods for identifying new selective progesterone receptor modulators for practicing disclosed methods of treatment.
Owner:APTALIS PHARMA
Treatment of prostate carcinoma
ActiveUS9132105B2Inhibiting and delaying growthReduce the amount requiredBiocideHydroxy compound active ingredientsMedicineProstate carcinoma
Owner:PELLFICURE PHARMA
Neutralizing prolactin receptor antibodies and their therapeutic use
Owner:BAYER IP GMBH
Combination therapy for the treatment of cancer
The present invention provides methods of treating cancer comprising treating a patient in need thereof with hormone therapy and administering to the patient a therapeutically effective amount of at least one metalloprotease inhibitor. The present invention also provides pharmaceutical compositions comprising a therapeutically effective amount of at least one hormone therapy agent, at least one metalloprotease inhibitor, and a pharmaceutically acceptable carrier.
Owner:INCYTE HLDG CORP
Novel treatment of prostate carcinoma
ActiveUS20150352060A1Inhibiting and delaying growthReduce the amount requiredBiocideHydroxy compound active ingredientsMedicineProstate cancer
Owner:PELLFICURE PHARMA
Chinese medicine preparation for treating importence
InactiveCN101584790BNo side effectsGood curative effectHeavy metal active ingredientsAnthropod material medical ingredientsDiseaseCnidium
The present invention provides a traditional Chinese medicine preparation for treating importence, which is the prepared slice comprising the following medicinal materials: 20-180 parts by weight of Astragalus root; 15-30 parts by weight of Chinese angelica; 15-30 parts by weight of pilose asiabell root or 15-20 parts by weight of ginseng; 15-30 parts by weight of white peony root; 6-15 parts by weight of actynolin; 2-3 parts by weight of centipede; 10-15 parts by weight of shorthorned epimedium herb; 10-15 parts by weight of dodder seed; 15-30 parts by weight of common cnidium fruit; 6-15 parts by weight of chuanxiong rhizome; 6-15 parts by weight of nutgrass galingale rhizome; 3-5 parts by weight of powdered antler or 0.5-1.5 parts by weight of hairy antler; and 6-10 parts by weight of liquorice. The Chinese medicine preparation for treating importenance according to the invention applies the method of general nourishment on the basis of the theory of traditional Chinese medicine forregulating the dysfunction of meridian of human organ and the unsmooth running of qi-blood and body fluid. The invention overcomes the defect of unremarkable effect when the method of temperature compensation is singly applied for treating the asynodia according to the traditional Chinese medicine. Simultaneously the side effect caused by singly adopting vasodilation or hormone therapy accordingto the western medicine. The traditional Chinese medicine of the invention has the characteristics of quick curative effect, no toxic or side effect, flexible theory and radical treatment. Simultaneously according to the principle of treating the root of the disease in the theory of traditional Chinese medicine, the medicine is added or reduced according to the illness state on the basis of general nourishment for adopting the symptoms of different patients or the illness states of a same patient in different periods.
Owner:李进才
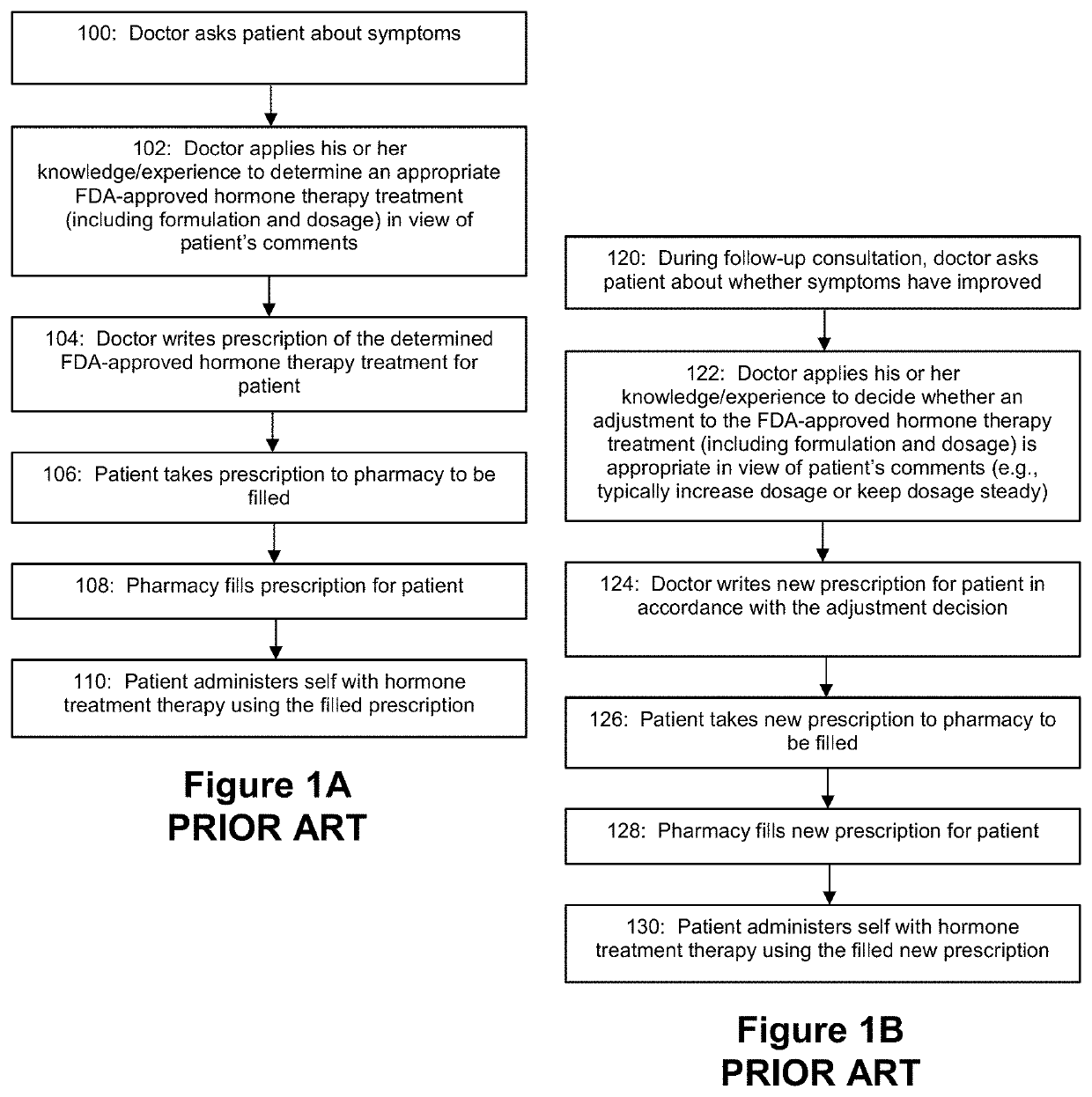
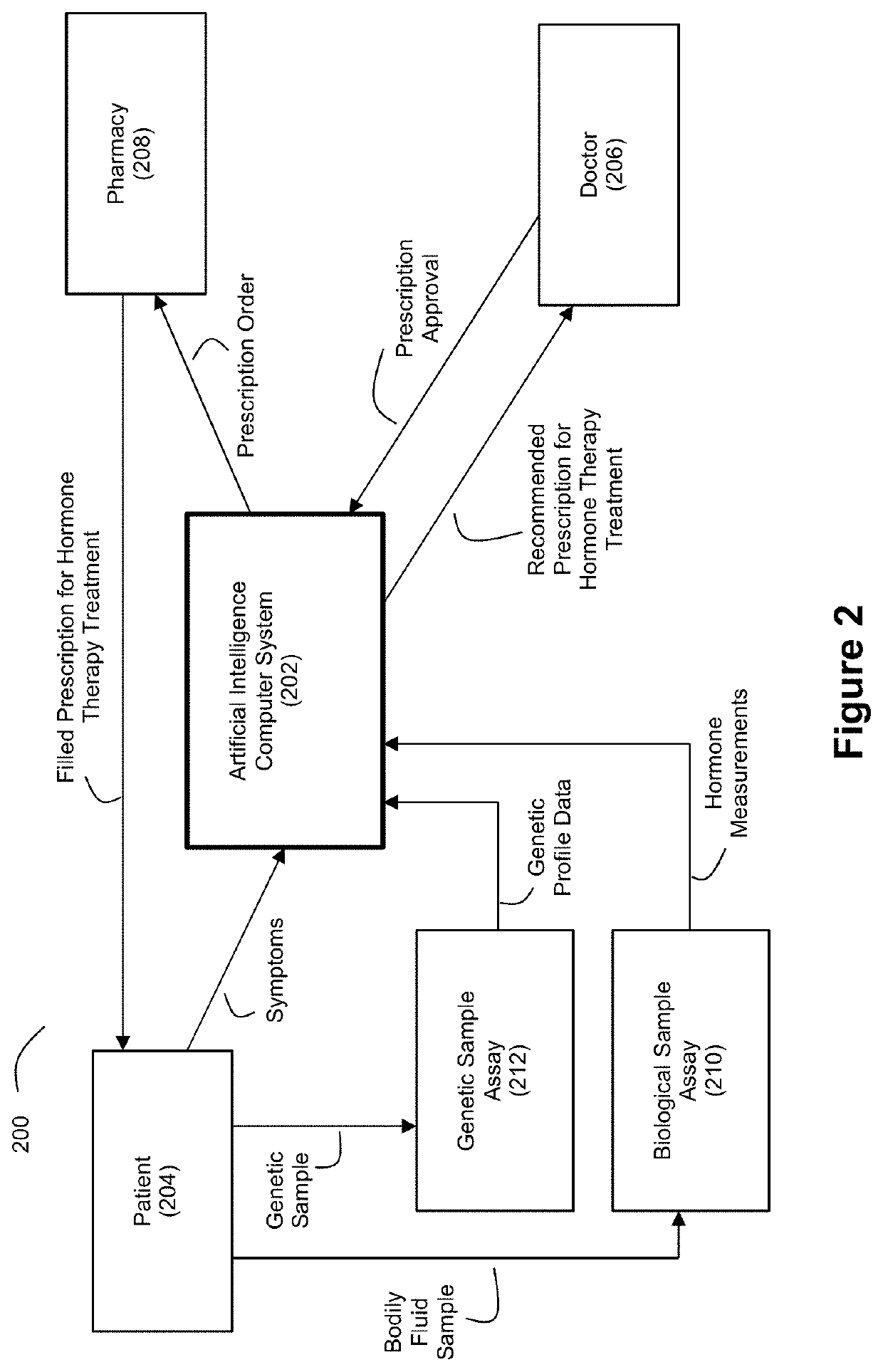
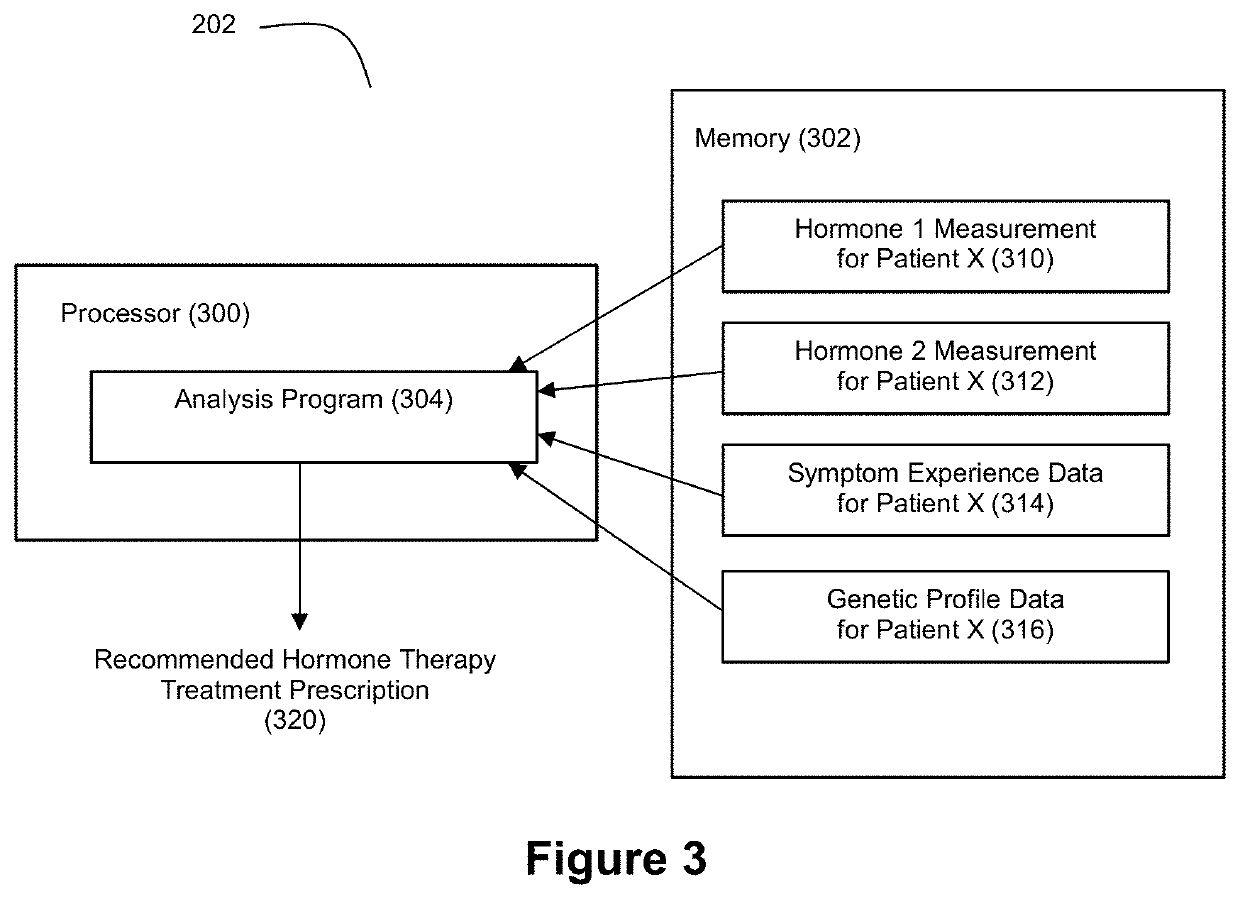
Applied Artificial Intelligence Technology for Hormone Therapy Treatment
ActiveUS20200118662A1Accurate trackingImprove personalizationPeptide/protein ingredientsHealth-index calculationPhysiologyHormonal therapy
Disclosed herein are a number of techniques that systematically integrate a person's biochemical, symptomatic, and genetic status to generate recommended hormone therapy treatment prescriptions.
Owner:NORMAN JAMES GLENN
Molecularly targeted combination drug for tumor treatment and prevention
InactiveUS20150352076A1Accelerate the accumulation processInhibit tumor growthBiocideAnimal repellantsCaspase pathwayTumor therapy
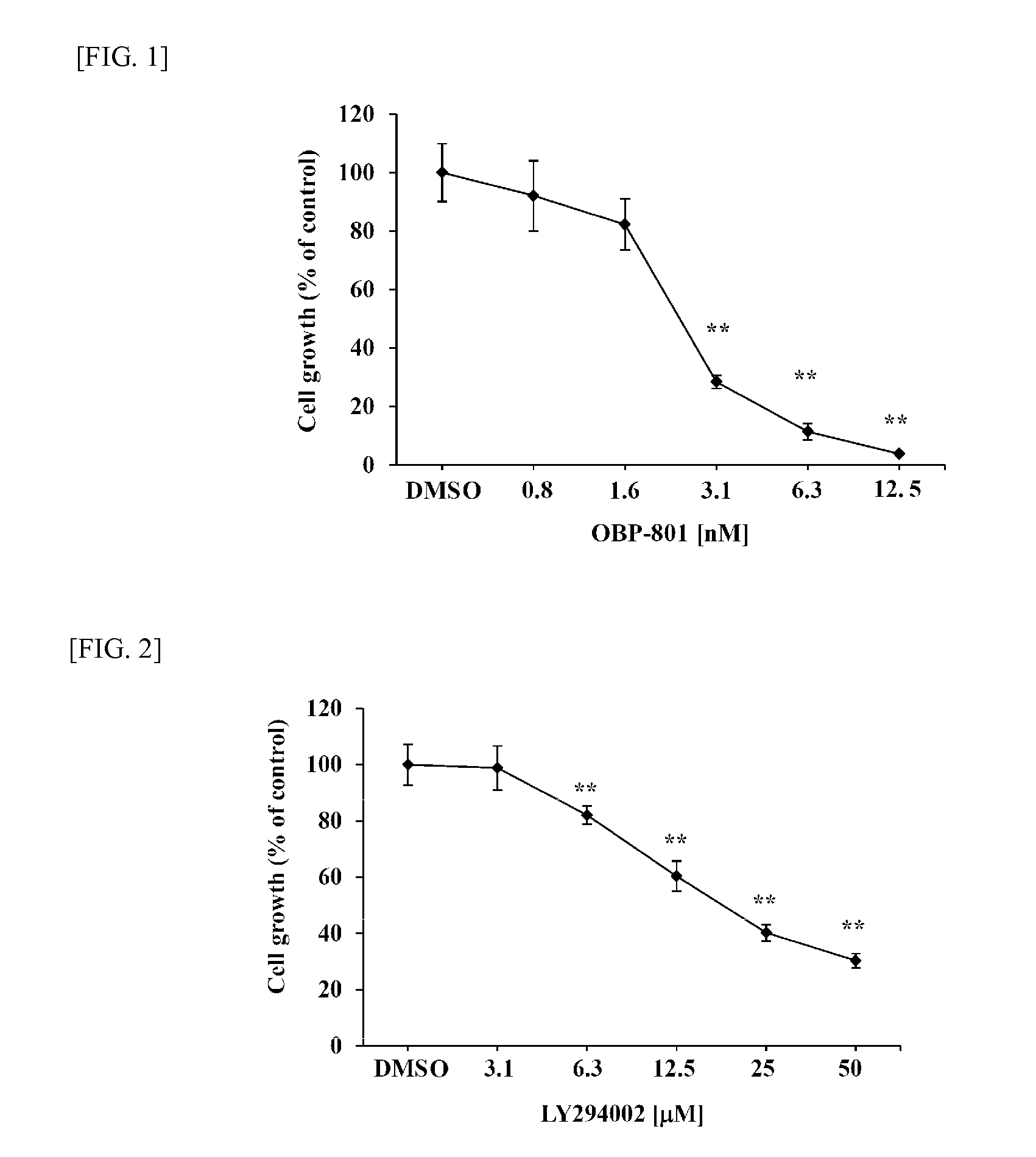
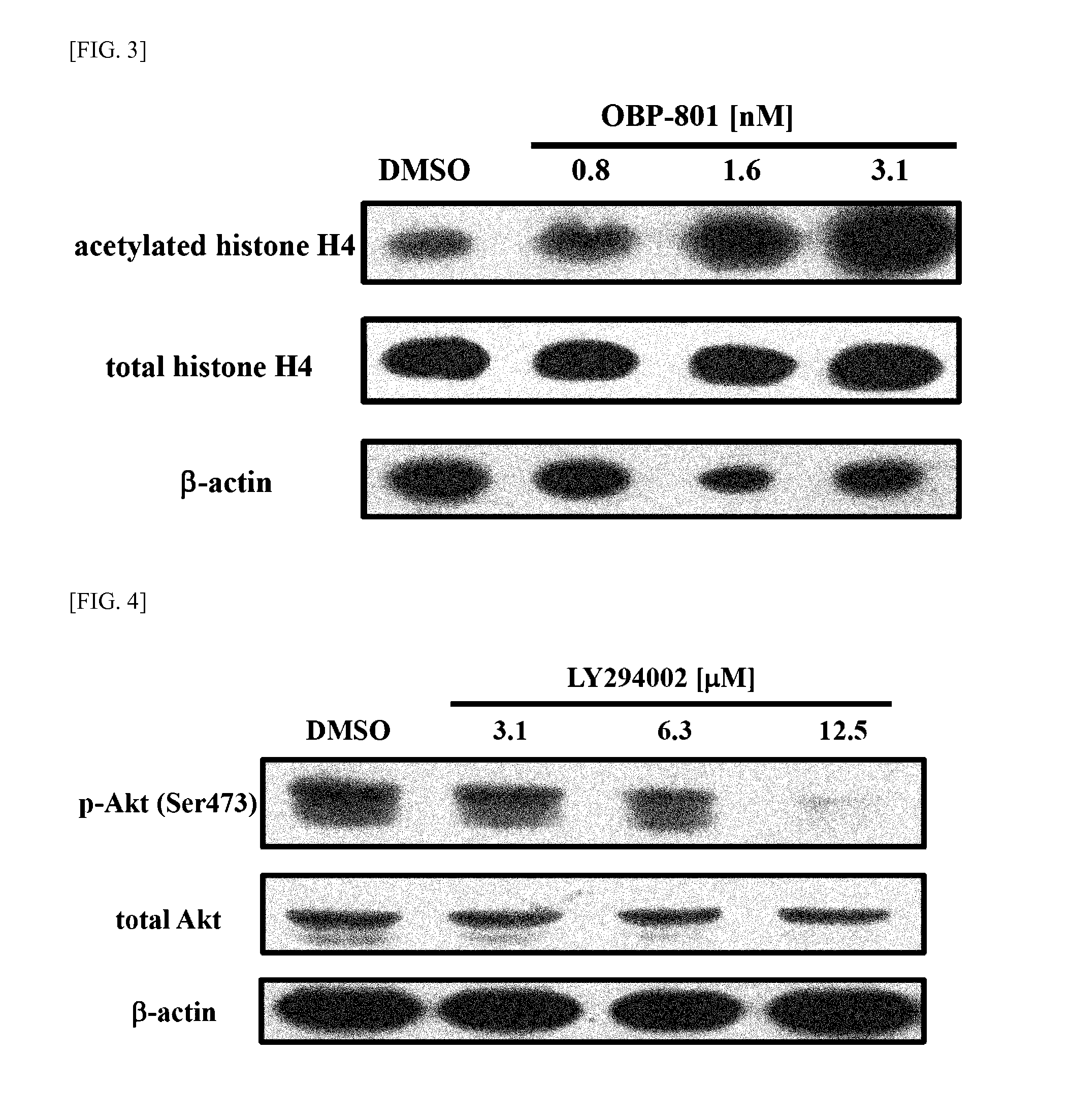
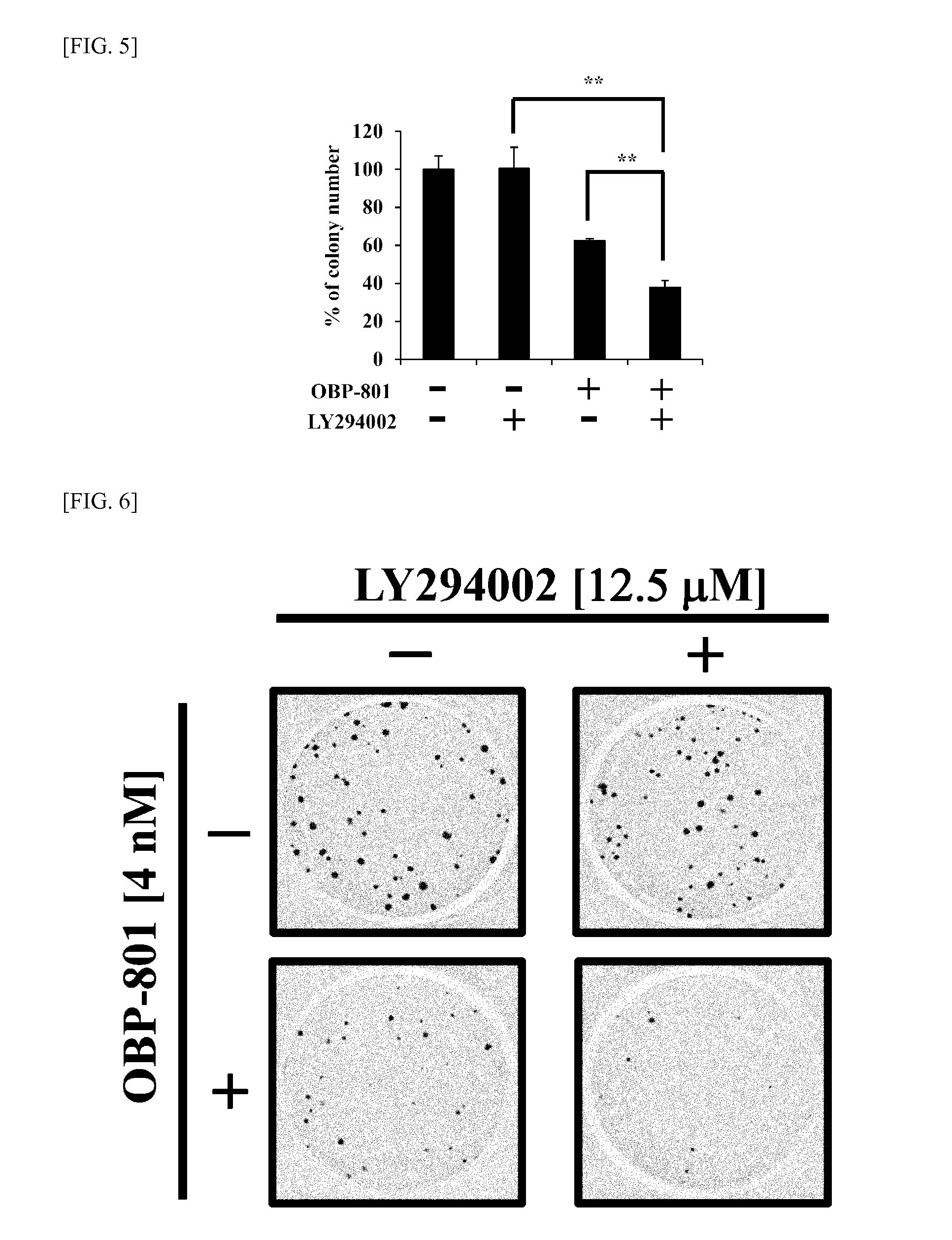
To provide means for treating and preventing a wide variety of cancers and tumors, including cancer in which there is PI3K / Akt pathway activation or p53 deactivation, or tumors for which chemotherapy, radiation therapy, hormone therapy, and other such conventional treatment methods have low effectiveness, different molecularly targeted drugs such as OBP-801 and PI3K inhibitor—preferably LY294002, BKM120, GDC-0941, BEZ235, BYL719, or CH5132799—are used in combination. This makes it possible to simultaneously obtain a plurality of different marked pharmaceutical benefits which are synergistic, not being obtainable through use of a formulation with either of the respective molecularly targeted drugs alone, such as caspase pathway activation, enhanced expression of Bim, increased accumulation of intracellular reactive oxygen species, and suppressed expression of survivin and XIAP protein, and makes it possible to provide new and clinically effective tumor treatment / prevention strategies.
Owner:SAKAI TOSHIYUKI +1
Medicament for treating hysteromyoma
InactiveCN102100876ASignificant effectNo side effectsAntineoplastic agentsSexual disorderSide effectCurcuma zedoaria
The invention discloses a medicament for treating hysteromyoma. The medicament mainly comprises the following components in part by weight: 20 to 30 parts of white peony root, 20 to 30 parts of red peony root, 15 to 20 parts of safflower, 20 to 25 parts of angelica, 15 to 20 parts of tuber fleeceflower, 20 to 30 parts of burreed tuber, 20 to 30 parts of zedoary, 20 to 30 parts of fresh aloe, 20 to 30 parts of prepared rehmannia root, 20 to 25 parts of medlar and 20 to 25 parts of herba lycopi leaf; and the medicament is prepared by a common method. Clinical medicament effect tests show that the medicament has the advantages of quick response, good effect, avoidance of operation, no pain, no wound, no side effect caused by hormone therapy and low treatment cost.
Owner:冯安华
Compositions and methods for suppressing endometrial proliferation
InactiveCN104997789AOrganic active ingredientsAntipyreticProgesteronesSelective progesterone receptor modulator
The subject matter of the instant invention is pertinent to the field of hormone therapy. More specifically, the subject matter of the instant invention concerns methods of treating estrogen-dependent conditions such as endometrial hyperplasia and endometrial cancer in a female undergoing estrogen and / or selective estrogen receptor modulator (SERM) therapy. The instant invention is also relevant to the suppression of endometrial proliferation. The instant invention is also relevant to the treatment of pain associated with endometriosis. The compositions for practicing the methods, comprising progesterone antagonists are also disclosed. Embodiments of the instant invention also disclose methods for identifying new selective progesterone receptor modulators for practicing disclosed methods of treatment.
Owner:APTALIS PHARMA
Neutralizing prolactin receptor antibodies and their therapeutic use
The present invention is directed to the neutralizing prolactin receptor antibody 006- H07, and antigen binding fragments, pharmaceutical compositions containing them and their use in the treatment or prevention of benign disorders and indications mediated by the prolactin receptor such as endometriosis, adenomyosis, non-hormonal female contraception, benign breast disease and mastalgia, lactation inhibition, benign prostate hyperplasia, fibroids, hyper- and normoprolactinemic hair loss, and cotreatment in combined hormone therapy to inhibit mammary epithelial cell proliferation. The antibodies of the invention block prolactin receptor-mediated signaling.
Owner:BAYER IP GMBH
Combination Hormone Therapy for the Eye
InactiveUS20110319370A1Reducing cataractReducing age-related macular degenerationOrganic active ingredientsBiocidePhysiologyAndrogen
An ophthalmic instillation composition which contains melatonin and at least one other hormone, typically an estrogen but also possibly testosterone or another aromatasable androgen. The instillation is designed to reduce or eliminate cataracts in men or women to which such an ophthalmic instillation composition is administered.
Owner:DUQUESNE UNIVERSITY
Placental extract biological gel preparation for treating premature ovarian failure and preparation method thereof
ActiveCN107375333AAvoid security issuesAvoid immune rejection problemsAerosol deliveryOintment deliveryGel preparationPhysiology
The invention discloses a placental extract biological gel preparation for treating premature ovarian failure and a preparation method thereof. The placental extract biological gel preparation is prepared from a gel substrate and placental extract serving as an active ingredient, wherein the volume of the placental extract is 90 to 99.5% of that of the biological gel preparation. According to the placental extract biological gel preparation disclosed by the invention, the placental extract is utilized as the active ingredient and obtained by being purified by multistep of technologies; not only are effective ingredients of collagen, polypeptide, varieties of growth factors and the like in placenta kept, but also no cell component is contained, and the problems of safety and immunological rejection of cell preparation are solved. The preparation is favorable for promoting ovarian function reconstruction of a premature ovarian failure patient, has a very good premature ovarian failure treating effect, avoids oral hormone therapy and hormone dependence, utilizes mucosa transdermal absorption playing action, can be fully absorbed, avoids injection and has better safety.
Owner:GUANGZHOU ZHUNYOU BIOLOGICAL TECH CO LTD
Composition for prostate health
InactiveUS8354126B1Toxic reductionReduce in quantityBiocideHydrocarbon active ingredientsDiseaseSaw palmetto extract
The present invention provides an effective, all-natural, non-toxic, non-hormonal composition consisting of vitamin D3, vitamin E, selenium, green tea extract, saw palmetto berry extract, isoflavanoids, and lycopene for prostate health. The invention provides compositions and methods to prevent, alleviate, and / or treat symptoms associated with prostate conditions and diseases. The prostate health composition may be used to supplement medical treatment such as radiation therapy, chemotherapy, and hormone therapy.
Owner:ONCONATURAL SOLUTIONS
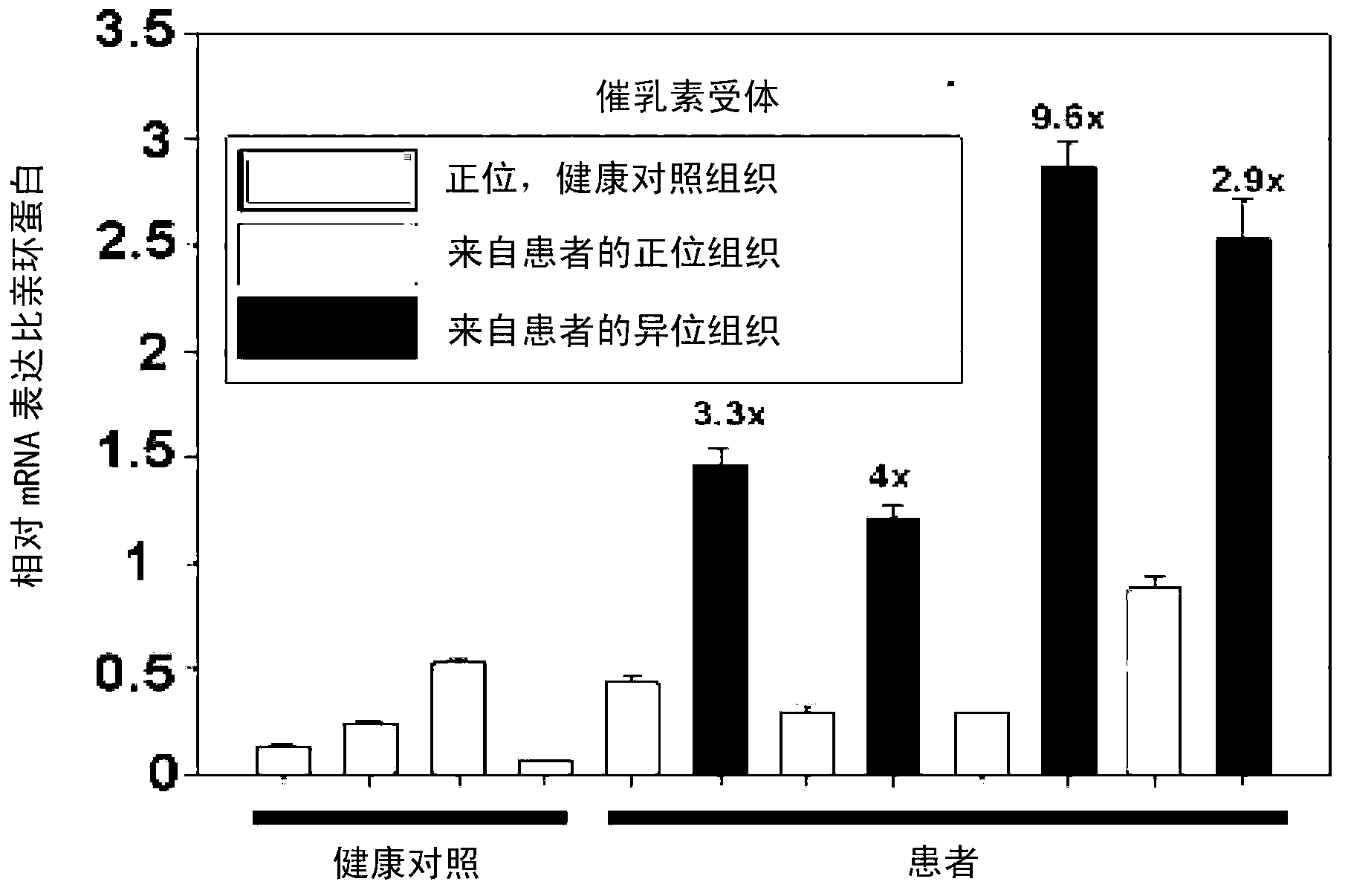
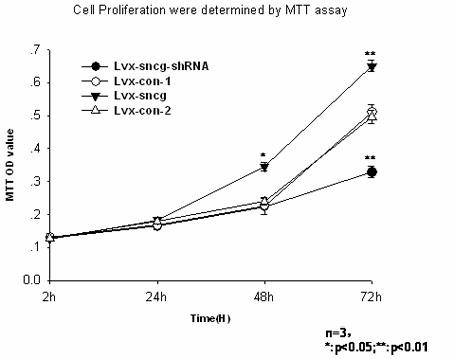
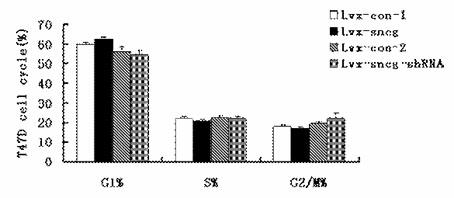
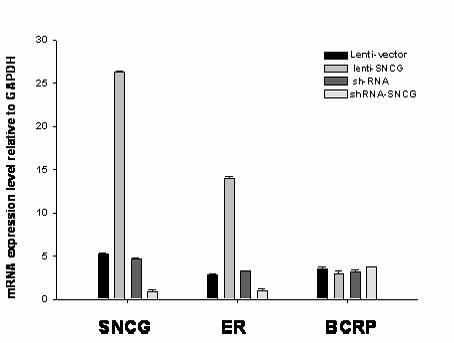
Application of synuclein gamma (SNCG) gene in diagnosis and hormone therapy of breast cancer
InactiveCN102586407AOpen up new ideasRaise the possibilityGenetic material ingredientsMicrobiological testing/measurementEstrogen receptorExpression gene
The invention relates to the application of synuclein gamma (SNCG) gene in diagnosis and hormone therapy of breast cancer, and the hormone therapy of breast cancer can be influenced by SNCG gene through adjusting the activated state of estrogen receptor (ER). The invention also relates to the application of the SNCG gene in the preparation of products for diagnosing the breast cancer. The invention has the advantages that the SNCG gene expression can be used for adjusting ER and hormone therapy sensibility, so that a new thought is created for the hormone therapy of the breast cancer; the SNCG gene expression can be used for adjusting ER and hormone therapy sensibility, so that the possibility of developing a new medicine for treating the breast cancer can be improved; and the SNCG gene can be used as a specific marker gene for diagnosing the breast cancer, so that the breast cancer can be more accurately and rapidly diagnosed.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com