Application of ILF3 detection reagent in preparation of colorectal cancer diagnosis reagent
A technology for detection reagents and detection kits, which is applied in the field of colorectal cancer prognostic diagnostic reagents/kits, and can solve problems such as unsatisfactory treatment of CRC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Patient and tissue samples
[0084] Fresh paired frozen samples of primary colorectal cancer and adjacent normal colon tissue were extracted from the Department of Surgery of the Sixth Affiliated Hospital of Sun Yat-sen University. All patients had stage II or III disease at the time of sample collection.
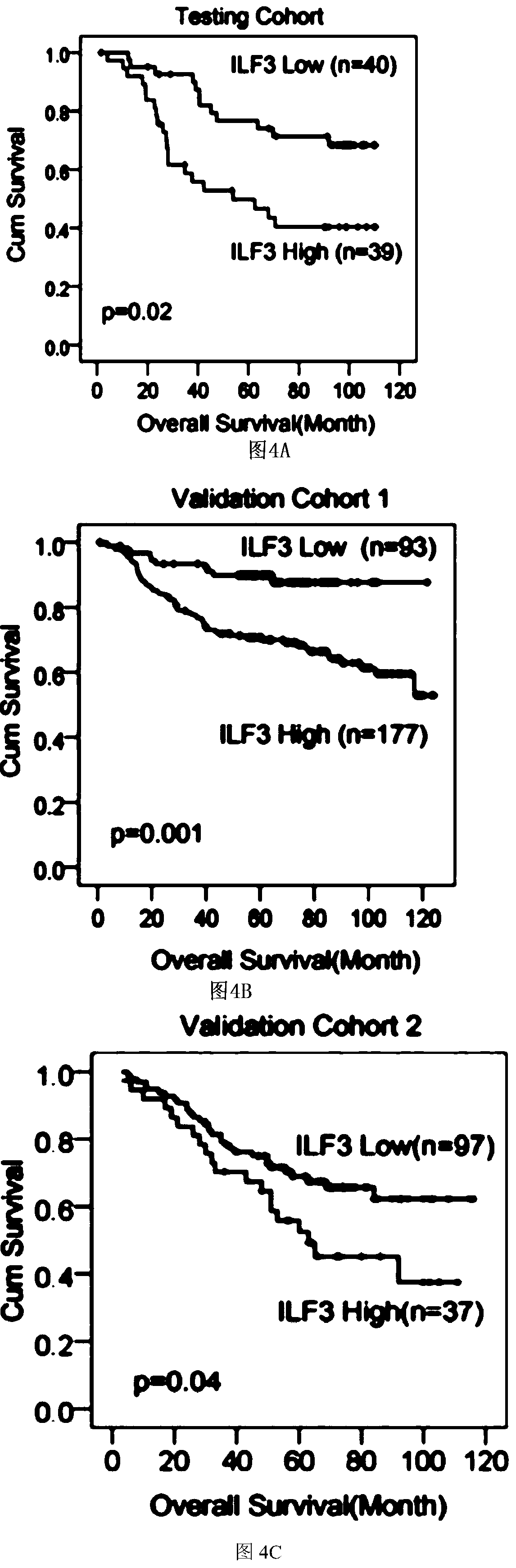
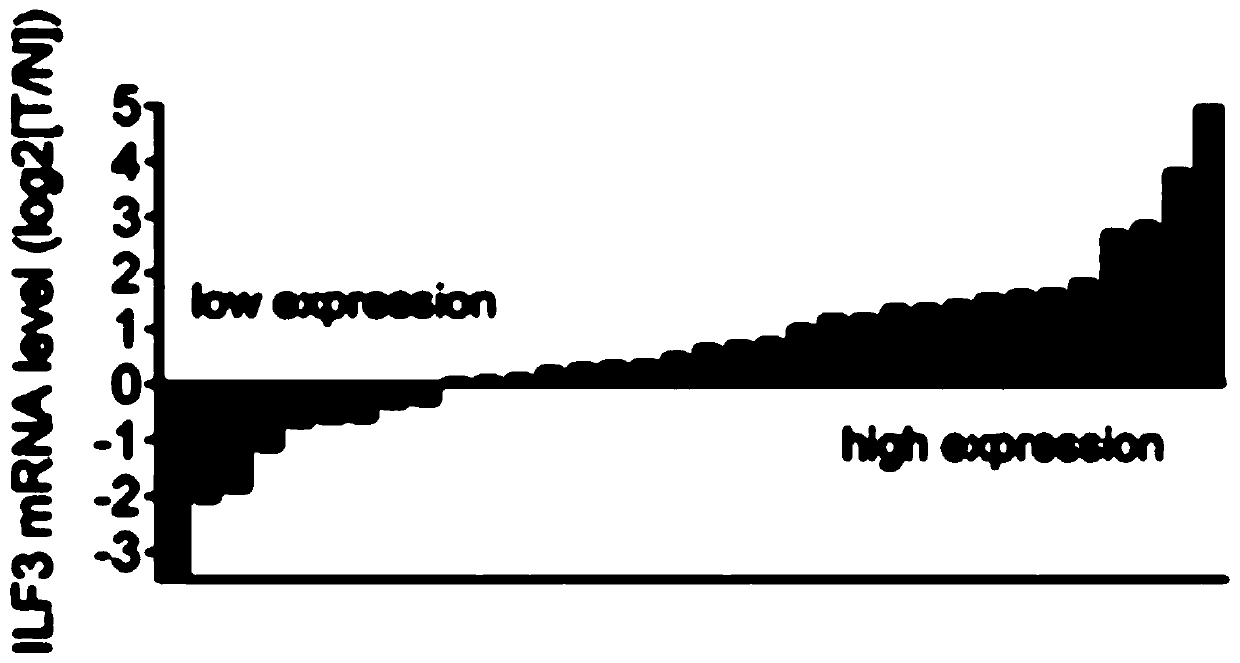
[0085] Additionally, paraffin-embedded samples of primary colorectal adenocarcinoma (prepared as TMA) were obtained from three independent cohorts of CRC patients: (1) 79 patients in the Sixth Affiliated Hospital of Sun Yat-sen University (test cohort); ( 2) 270 patients from the First Affiliated Hospital of Sun Yat-sen University (validation cohort 1); (3) 134 patients from the 150th Central Hospital of the Chinese People's Liberation Army (validation cohort 2). Raw immunohistochemistry slides were scanned by Aperio Versa (Leica Biosystems), which captured digital images of immunostained slides. Genie calculates H-scores for regions selected by pathologi...
Embodiment 2
[0086] Example 2 Cell Culture, Reagents and Transfection
[0087] All cells were obtained from ATCC and stored at 37 °C and 5% CO 2 condition. Among them, DLD-1 and HCT-8 cells were kept in RPMI 1640 medium (RPMI) containing 10% (v / v) fetal bovine serum (FBS). 293T, RKO, HT29, WiDR and SW620 cells were cultured in DMEM medium (containing 10% FBS). All transient transfections of plasmid and siRNA-introduced cell lines followed the instructions of Lipofectamine 2000 Transfection Reagent (Thermo Fisher, #11668019).
Embodiment 3
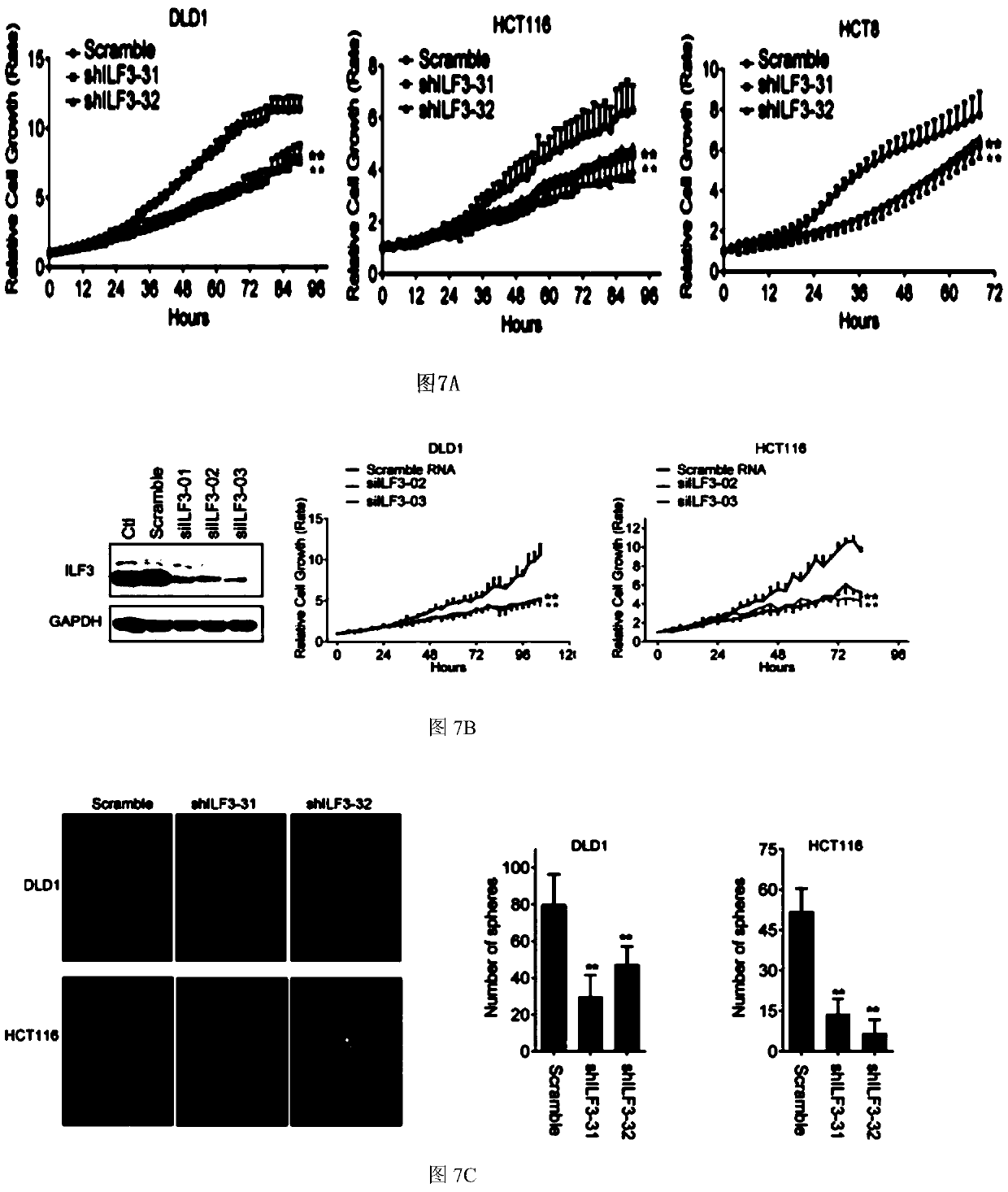
[0088] The shRNA knockdown of embodiment 3ILF3
[0089] The present invention screens four hairpin-type shRNAs against human ILF3 transcripts, and finds two independent sequences that can reduce mRNA levels by >70%. The shRNAs are in the pLKO.1 vector (#31 and #32). #35 was designed to target the 3'UTR of ILF3.
[0090] The steps for preparing lentiviral particles are as follows: the 55cm 2 1 x 10 in dish 7 HEK293T cells were co-transfected with 10 μg pLKO.1 shRNA construct, 5 μg psPAX2 and 5 μg pMD2.G. 48 and 72 hours after transfection, the supernatant containing virus particles was collected and filtered through Millex-GP filters (0.45 μm pore size, Millipore). To infect cancer cells with lentivirus, cells were infected twice with a medium containing 2 mL lentivirus, 200 μL FBS, and 5 mg / mL polybrene (Sigma) at 37° C. for 24 hours and 48 hours. To increase knockdown efficiency, puromycin selection was performed on infected cells for several days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com