A recombinant antibody against human cardiac troponin I
A technology of cardiac troponin and binding protein, which is applied in the field of immunity and can solve the problems of low activity and poor affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] This example provides an exemplary preparation method of a recombinant antibody against human cardiac troponin I.
[0120] S10, construction of expression plasmids:
[0121] In this embodiment, restriction endonuclease and Prime Star DNA polymerase were purchased from Takara Company;
[0122] MagExtractor-RNA extraction kit was purchased from TOYOBO;
[0123] BD SMART™ RACE cDNA Amplification Kit was purchased from Takara;
[0124] The pMD-18T vector was purchased from Takara;
[0125] The plasmid extraction kit was purchased from Tiangen Company;
[0126] Primer synthesis and gene sequencing were completed by Invitrogen;
[0127] Secreted Anti-cTnI 12D2 monoclonal antibody was used as an existing hybridoma cell line, which was revived for later use.
[0128] S11, design and synthesis of primers:
[0129] 5' RACE upstream primers for amplification of heavy and light chains:
[0130] SMARTER II A Oligonucleotide:
[0131] 5'>AAGCAGTGGTATCAACGCAGAGGTACXXXXX<3';
...
Embodiment 2
[0151] Transient Transfection of Recombinant Antibody Expression Plasmids into CHO Cells and Identification of Antibody Activity in Expression Supernatant
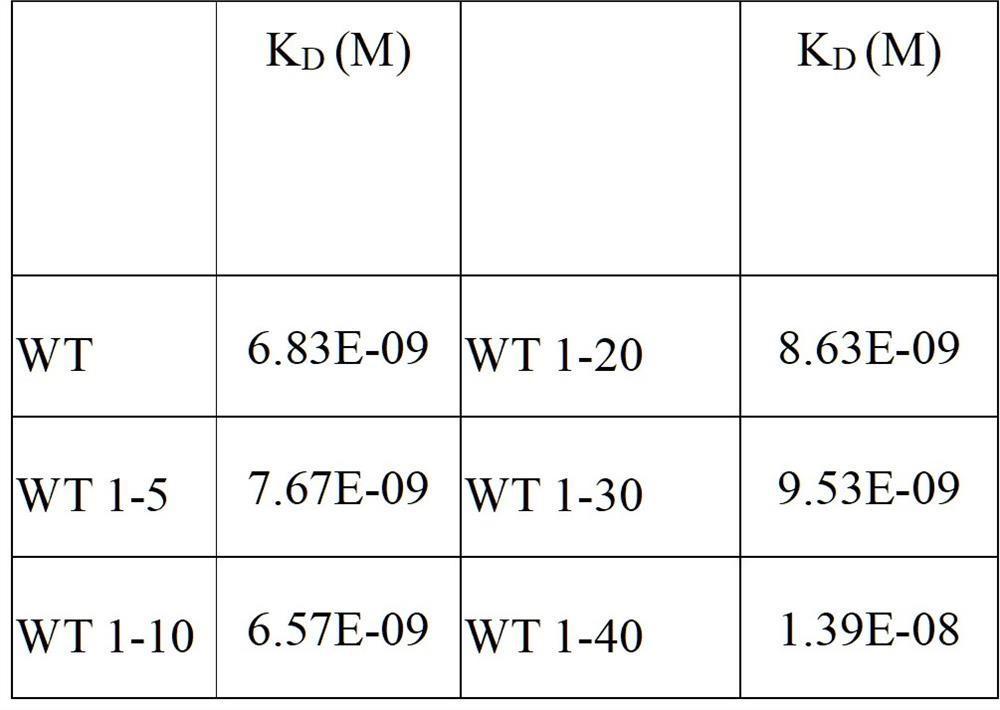
[0152] The plasmid was diluted to 400ng / ml with ultrapure water, and the CHO cells were adjusted to 1.43×10 7 cells / ml in a centrifuge tube, mix 100ul plasmid with 700ul cells, transfer to an electroporation cup, electroporation, transfer to 10ml CD CHO AGT medium, culture in a 37-degree shaker (8% CO 2 , amplitude 150); sampling the cell viability every day, when the cell viability is lower than 50%, centrifuge the cell culture supernatant, and the obtained antibody (with sequences such as light chain and heavy chain shown in SEQ ID NO:11 and 12 ). Take 4µg of purified antibody for reducing SDS-PAGE.
[0153] After analysis, the complementarity determining region (WT) of the heavy chain:
[0154] CDR-VH1 is G-F(X1)-T-F-T-E(X2)-Y-N-V(X3)-H;
[0155] CDR-VH2 is Y-I(X1)-Y-P-N(X2)-N-G-I-S(X3)-G-Y-N-Q;
[0156] CDR-VH3 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com