Catalyst, preparation method thereof and method for preparing lower olefin from synthesis gas
A catalyst and synthesis gas technology, applied in molecular sieve catalysts, chemical instruments and methods, and hydrocarbon production from carbon oxides, etc., can solve problems such as unusable catalysts, and achieve improved catalytic activity, high raw material conversion rate and target product selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
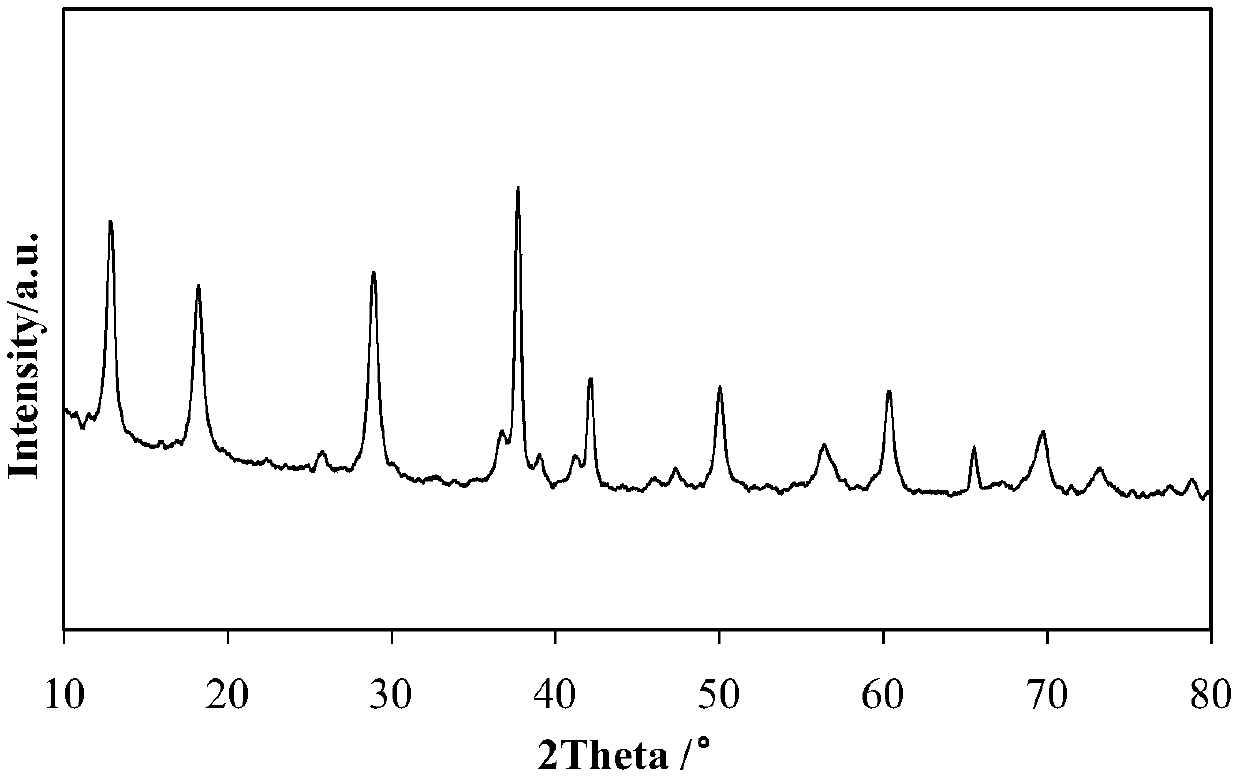
[0060] Dissolve 3.17g potassium permanganate in 40.55g deionized water, heat and stir to dissolve it to form potassium permanganate solution, add 1.16g ferric nitrate to 3.64g of 50% by weight manganese nitrate solution and stir evenly to obtain ferric nitrate containing manganese nitrate solution, the weight ratio of potassium permanganate to manganese nitrate is 1.7:1, and the molar ratio of Fe:Mn is 1:10. The above two solutions are mixed and transferred to a hydrothermal reaction kettle, and placed in 180°C water Thermal reaction 24h. The resulting brown precipitate was washed with deionized water several times until the pH of the washing solution was 7, and then the solid product was dried at 120°C overnight and calcined at 400°C for 4 hours to obtain the catalyst A1 prepared in this example. The composition of Catalyst A1 is 8% by weight of Fe-OMS-2 based on the dry basis weight of .
Embodiment 2
[0062] Dissolve 3.17g potassium permanganate in 40.55g deionized water, heat and stir to dissolve it to form potassium permanganate solution, add 0.58g ferric nitrate to 3.64g of 50% by weight manganese sulfate solution and stir evenly to obtain ferric nitrate containing The manganese sulfate solution, the weight ratio of potassium permanganate and manganese sulfate is 1.7:1, the molar ratio of Fe:Mn is 1:20, the above two solutions are mixed, and transferred to a hydrothermal reaction kettle, in 180 ℃ water Thermal reaction 24h. The resulting brown precipitate was washed several times with deionized water until the pH of the washing solution was 7, and then the solid product was dried at 120°C overnight and calcined at 400°C for 4 hours to obtain the catalyst A2 prepared in this example. The composition of Catalyst A2 was 4% by weight Fe-OMS-2 based on the dry weight of .
Embodiment 3
[0064]Dissolve 3.17g potassium permanganate in 40.55g deionized water, heat and stir to dissolve it to form potassium permanganate solution, add 0.25g ferric nitrate to 3.64g of 50% by weight manganese nitrate solution and stir evenly to obtain ferric nitrate containing manganese nitrate solution, the weight ratio of potassium permanganate to manganese nitrate is 1.7:1, and the molar ratio of Fe:Mn is 1:25. The above two solutions are mixed and transferred to a hydrothermal reaction kettle, and heated in 180°C water Thermal reaction 24h. The resulting brown precipitate was washed with deionized water several times until the pH of the washing solution was 7, and then the solid product was dried at 120°C overnight and calcined at 400°C for 4h to obtain the catalyst A3 prepared in this example. The composition of Catalyst A3 was 3.2% by weight of Fe-OMS-2 based on the dry basis weight of .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com