Compositions and methods for inducing humoral and cellular immunities against tumors and cancer
A composition and cancer technology, applied in chemical instruments and methods, drug combinations, anti-tumor drugs, etc., can solve problems such as weak immunogenicity and shrinking immune response specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258] Tumor generation in mice
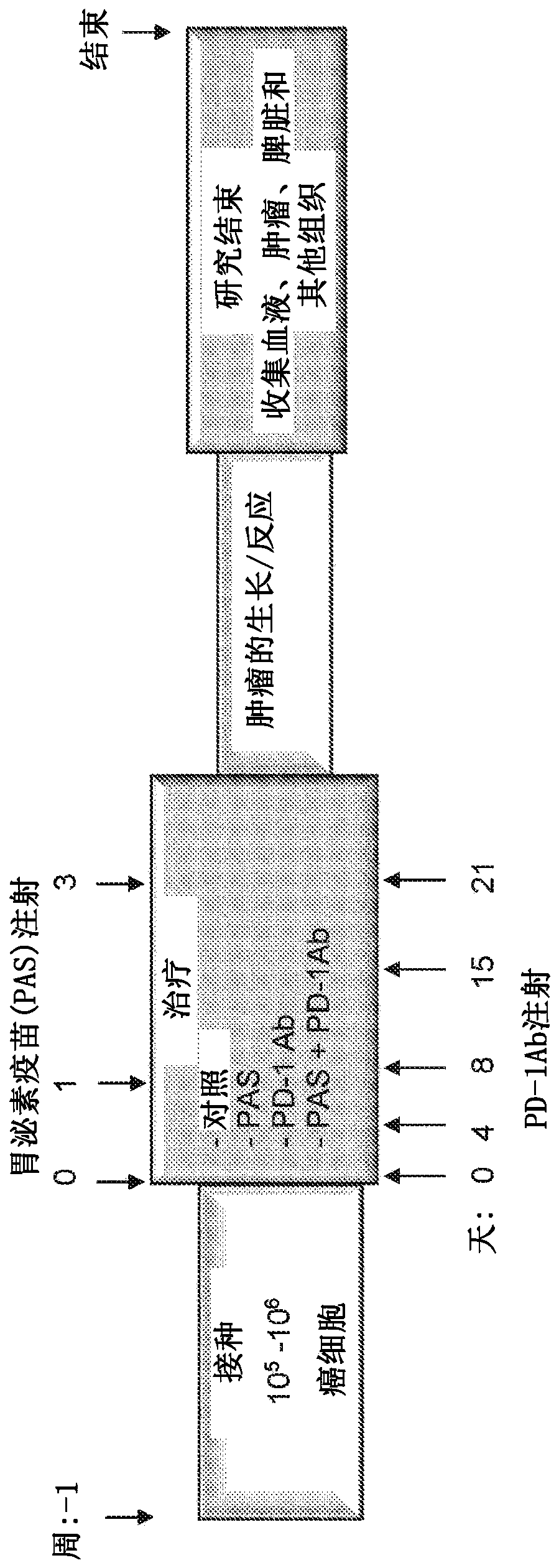
[0259] To determine whether PAS treatment induces both humoral and cellular immune responses and confers a synergistic effect on immune checkpoint antibody therapy, 5 x 10 5 A solution of 0.1 ml of murine mT3 pancreatic cancer cells in PBS is introduced subcutaneously into the flank to generate tumors in immunocompetent mice (eg, C57BL / 6 mice that are syngeneic for murine mT3 pancreatic cancer cells). One week after tumor formation, mice were treated with PAS and one or more immune checkpoint inhibitors, such as figure 1 shown.
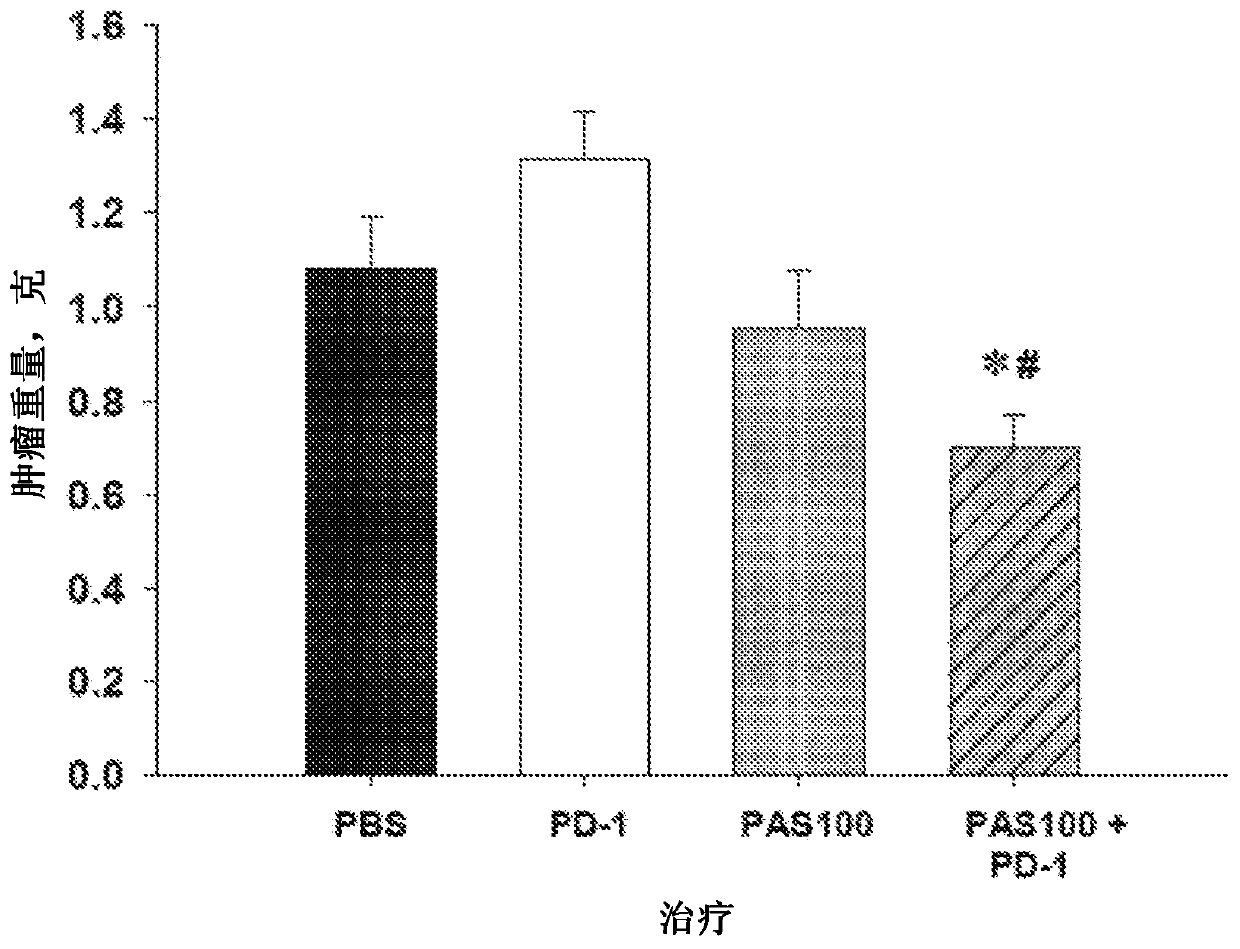
[0260] Treatment of animals was initiated one week after mT3 pancreatic cancer cell inoculation, as this time frame ensured that all animals in the study had palpable subcutaneous tumors and that the treatment did not interfere with tumor initiation. The primary endpoints were tumor growth and survival. Tumor growth was measured weekly with calipers and the volume of the tumor was calculated as L x W 2 x 0.5. ...
Embodiment 2
[0269] T in terminally differentiated CD3 EMRA CD4 – / CD8 – analysis of cells
[0270] T cell subsets
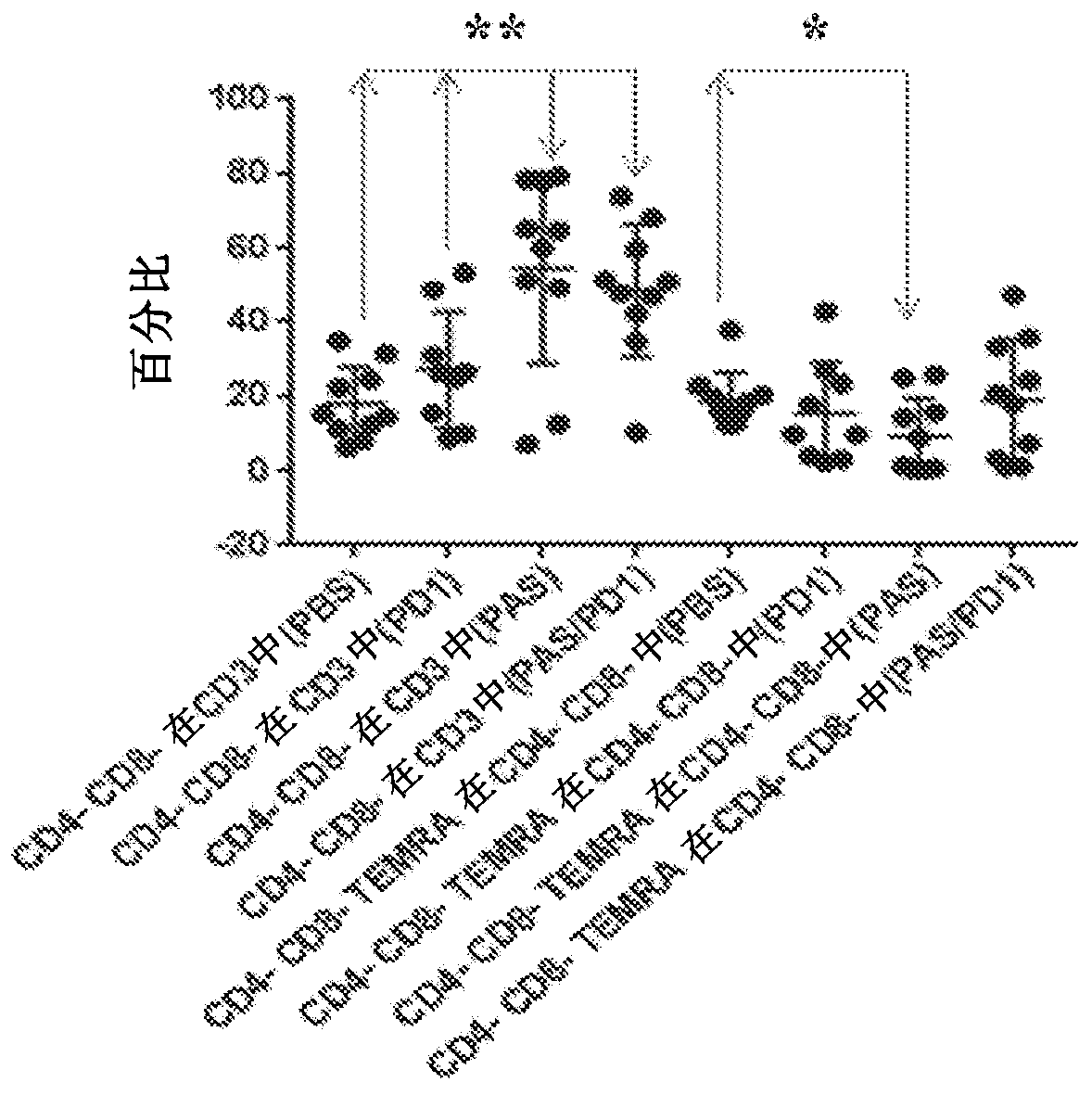
[0271] Tumors were induced in mice as described in Example 1. T lymphocytes were isolated from splenic peripheral blood mononuclear cells (PBMCs) isolated from mice treated with PBS, PD-1Ab, PAS100, or PAS100+PD-1Ab. Various subsets of T cells were identified by flow cytometry using the antibodies listed in Table 1. In particular, the first T cell subset, the CD3 + / CD4 – / CD8 – , and from this subset were then isolated representative CD3 + / CD4 – / CD8 – / CD44 – / CD62L – the T EMRA Another subpopulation of cells. The percentages and ratios of these various subpopulations present in mice that had been treated with PBS, PD-1Ab, PAS100, or PAS100+PD-1Ab have been determined and the results are shown in Figure 3A and Figure 3B middle.
[0272] Figure 3A CD3 in mice treated with PBS, PD-1Ab, PAS100 or PAS100 / PD-1 is shown + T in T cells EMRA cells (C...
Embodiment 3
[0275] Cytokine Activation Assay for PAS100
[0276] T lymphocytes were isolated from splenic peripheral blood mononuclear cells (PBMCs) isolated from mice treated with PAS100. These cells were evaluated by flow cytometry to determine whether they were indeed T cells activated by the cytokines activating interferon-gamma (INFG), granzyme-B (granzyme), perforin, and tumor necrosis factor-alpha (TNFα). cell. result in Figure 4A and Figure 4B available in .
[0277] Figure 4A showed that T cells isolated from mice treated with PAS100 were indeed activated. When these same cells were restimulated in culture with gastrin for 6 hours (see Figure 4B ), they were re-stimulated and released more cytokines, confirming immunization of PAS100-stimulated T cells and further confirming that these T cells specifically responded to gastrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com