Enhancer for t-cells or b-cells having memory function, malignant tumor recurrence inhibitor, and inducer for inducing memory function in t-cells or b-cells
A malignant tumor, B cell technology, applied in receptors/cell surface antigens/cell surface determinants, medical preparations containing active ingredients, antitumor drugs, etc., can solve the loss of function, side effects, unclear recurrence of malignant tumors and other problems to achieve the effect of inhibiting recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149]
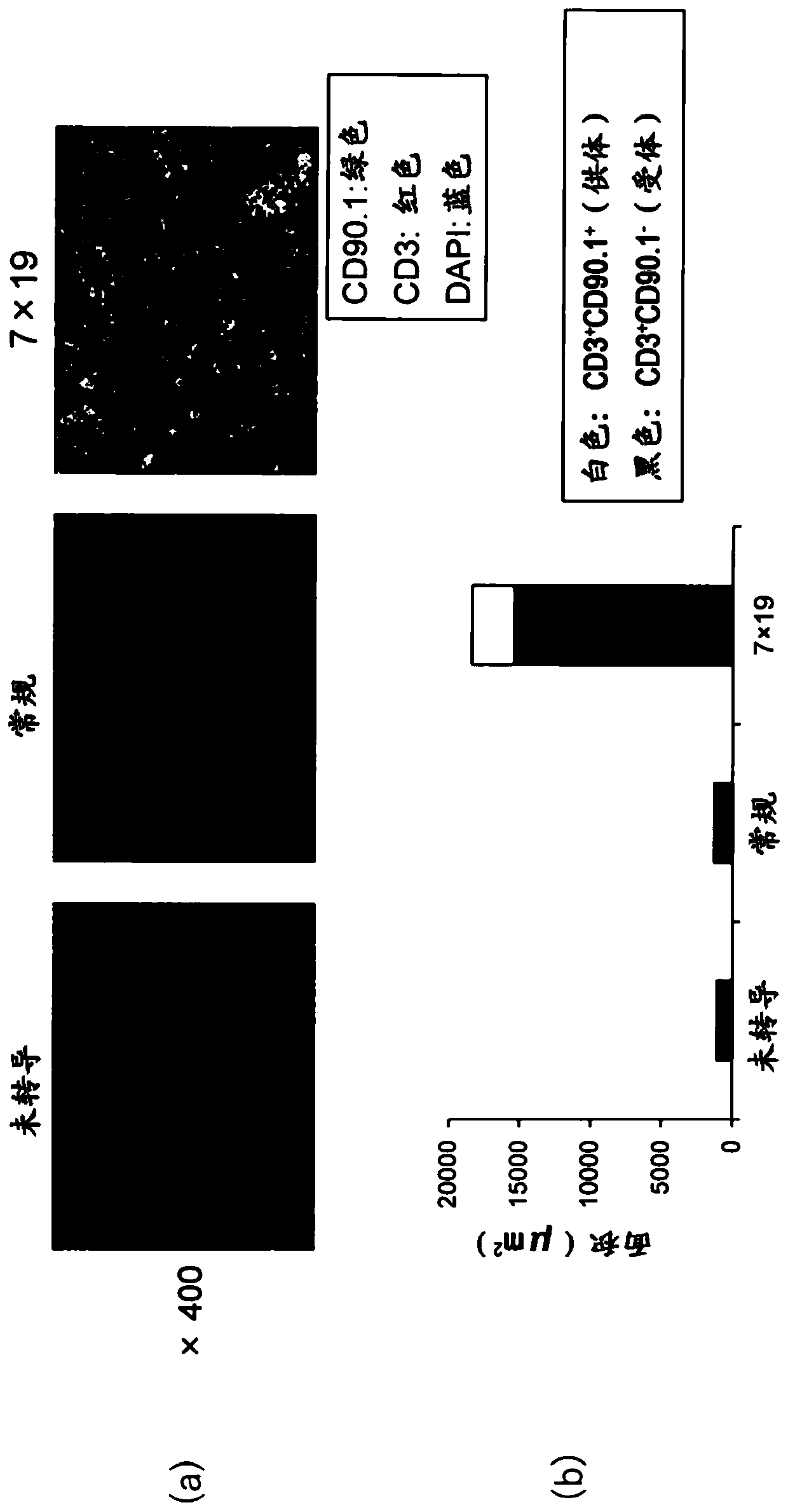
[0150] In order to investigate the anti-tumor effect of CAR-IL-7 / CCL19 expressing T cells, it was investigated whether endogenous T cells derived from the same host (recipient) as the administered donor T cells infiltrated in the tumor tissue. tumor tissue. First, 2.5×10 6 3LL-hCD20 (3LL derived from mouse lung cancer cells genetically recombined to express human CD20) (day 0). On the 7th day thereafter, cyclophosphamide (CPA: 100 mg / kg) as an anticancer agent was intraperitoneally administered. On day 10, positive CD90.1 (CD90.1 + ), CD90.2 negative (CD90.2 - ) 1×10 generated from congenic mice 6 The above-mentioned anti-human CD20 CAR-expressing T cells, the above-mentioned anti-human CD20 CAR-IL-7 / CCL19-expressing T cells, or the above-mentioned isolated mouse T cells without gene introduction were administered intravenously. On day 19, tumor tissues were removed from the mice. For primary staining, a biotin-labeled anti-CD90.1 antibody (cloneOX-7 BioLegend...
Embodiment 2
[0153]
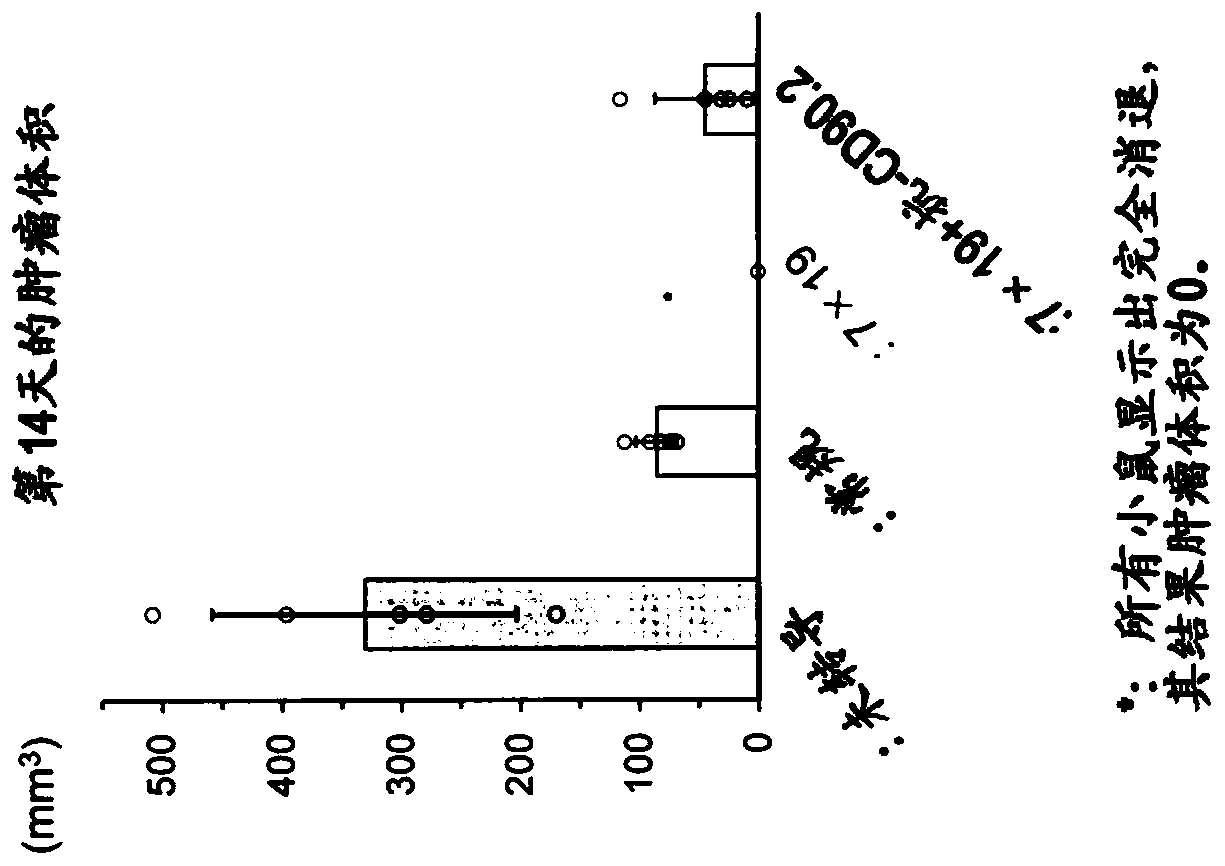
[0154] C57BL / 6 mice were subcutaneously inoculated with 2.5×10 6 3LL-hCD20 (day 0). On day 3 thereafter, positive CD90.1 (CD90.1 + ), CD90.2 negative (CD90.2 - ) 1×10 generated from congenic mice 6 Anti-human CD20 CAR-expressing T cells (Conventional: Conv.) or anti-human CD20 CAR-IL-7 / CCL19-expressing T cells (7×19) were administered intravenously. The antibody against CD90.2 (Thy1.2) expressed in endogenous T cells, that is, anti-CD90.2 antibody (anti-CD90.2: it is a hybridoma purchased from ATCC by the inventors of the present application (hybridoma)) was administered intraperitoneally twice a week from day 1. The dose was set to 1 mg / mouse for the first two doses, and 0.5 mg / mouse thereafter. The mean±SD of tumor volume on day 14 of each group (n=5) is shown in figure 2 . ○ represents the value of each mouse.
[0155] like figure 2 As shown, in the case of administration of anti-human CD20 CAR-IL-7 / CCL19 expressing T cells, the proliferation of malign...
Embodiment 3
[0157]
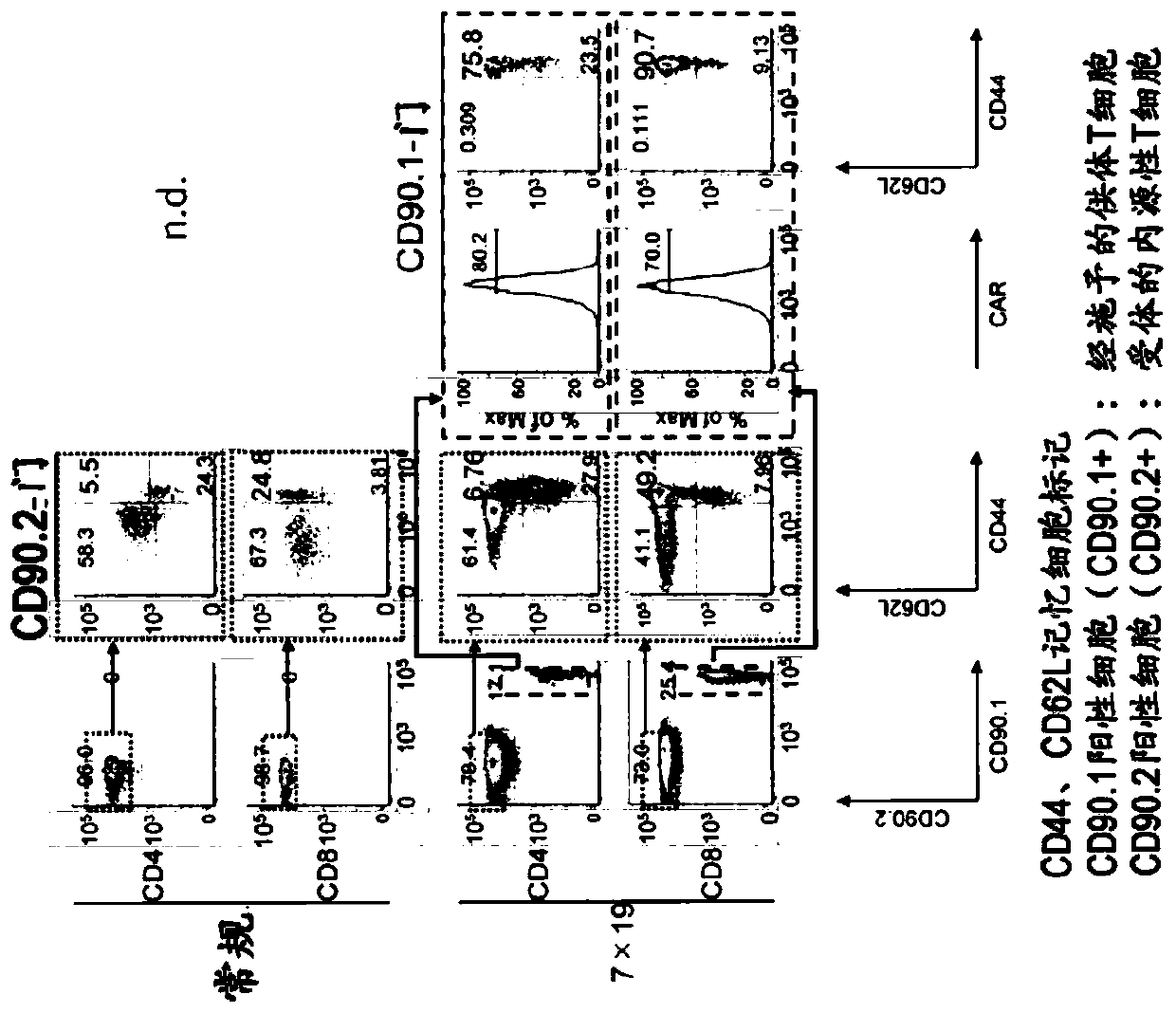
[0158] In terms of the acquisition of memory functions of donor CAR-T cells and endogenous T cells based on anti-human CD20 CAR-IL-7 / CCL19 expressing T cells, and the increase of cells with memory functions, the use of memory cells The markers of CD44 as well as CD62L were evaluated.
[0159] C57BL / 6 mice were subcutaneously inoculated with 2.5×10 6 3LL-hCD20 (day 0). On day 3 thereafter, positive CD90.1 (CD90.1 + ), CD90.2 negative (CD90.2 - ) 1×10 generated from congenic mice 6 The above anti-human CD20 CAR-expressing T cells (Conv.) or the above-mentioned anti-human CD20 CAR-IL-7 / CCL19 expressing T cells (7×19) were administered intravenously. On day 28, spleen cells were harvested for the following analysis.
[0160] Donor T cells were used as CD90.1 positive cells (CD90.1 + ), the recipient T cells were identified as CD90.2 positive cells (CD90.2 + ) and identified. Expression of memory T cell markers (CD44 and CD62L) and CAR in CD4-positive and CD8-pos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com