Preparation method and composition of human butyrylcholinesterase
A technology for butyrylcholinesterase and human butyrylcholinesterase is applied in the field of preparation of human butyrylcholinesterase and can solve the problems of long cycle, low pH buffer system of anion exchange chromatography, low efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
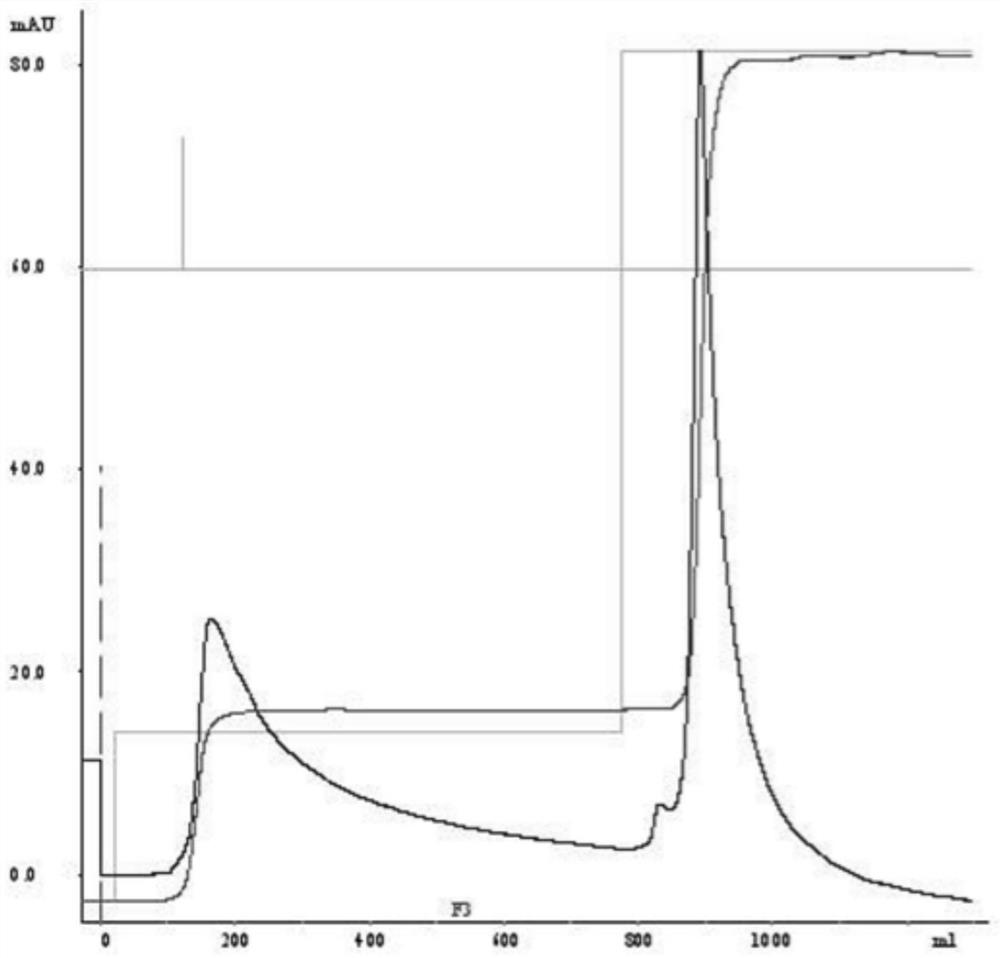
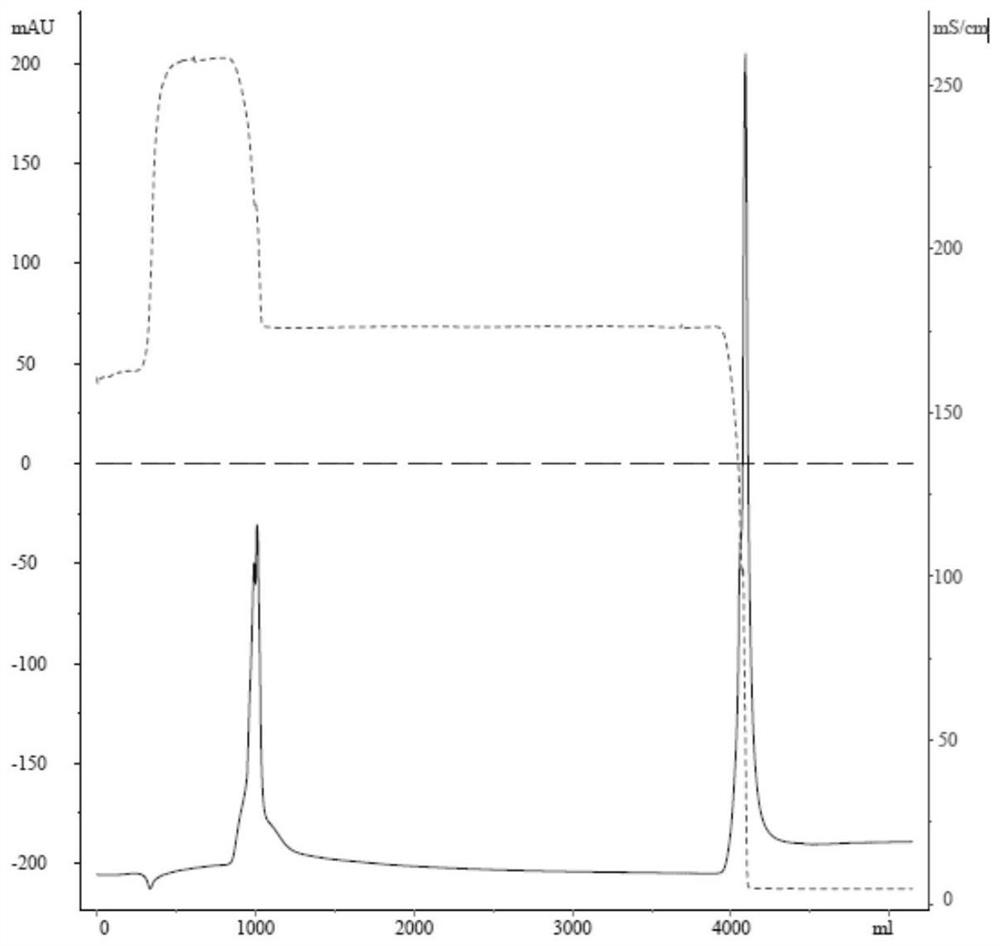
[0030] The embodiment of the present application provides a preparation method of human butyrylcholinesterase, including purification treatment, and the purification treatment includes:
[0031] The raw material of human butyrylcholinesterase is subjected to affinity chromatography, and the eluate of the target peak is collected;
[0032] performing ammonium sulfate salt-out on the target peak eluate collected by the affinity chromatography to obtain an ammonium sulfate precipitate;
[0033] The ammonium sulfate precipitate was subjected to hydrophobic chromatography, and the target peak eluate was collected.
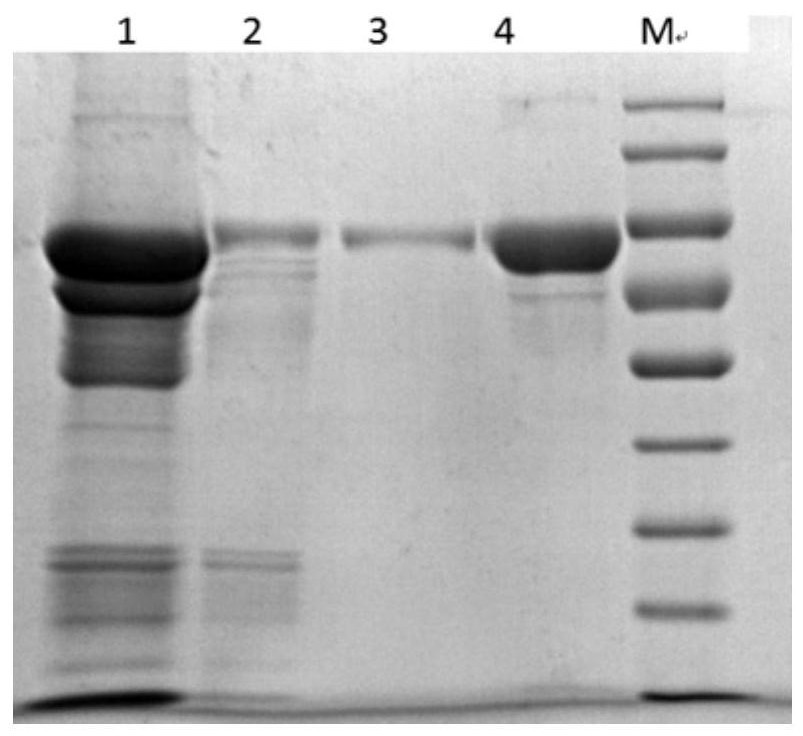
[0034] In the preparation method of human butyrylcholinesterase in the embodiment of the present application, the purification process with affinity chromatography, ammonium sulfate salting out and hydrophobic chromatography as the core is adopted, which can be applied to the production and preparation of HuBChE and recombinant HuBChE from different sources . The puri...
Embodiment 1
[0086] Embodiment 1: Take Cohn F-IV as raw material
[0087] The pretreatment of Cohn F-IV includes adjusting the pH value and ionic strength of Cohn F-IV, and removing protein precipitates and diatomaceous earth by pressure filtration. Then use organic solvent / detergent (S / D, 0.3% TNBP and 1% Tween-80) treatment method to inactivate lipid-enveloped viruses, etc., to further improve the safety of biological products.
[0088] Affinity chromatography purification process
[0089] Column parameters:
[0090] Affinity chromatography gel: the affinity ligand Procanamide is cross-linked with Sepharose CL-6B FF. Experimental glue 15-30L. Column diameter height: φ30×40cm. Flow rate: 200-2000ml / min. The temperature is 8-10°C.
[0091] A. Sample pretreatment
[0092] The supernatant after the pretreatment of the raw material Cohn F-IV was adjusted to pH 7.5±0.5 with NaOH solution, the conductance was 1±0.5ms, and the temperature was controlled at 8-10°C. Adjust the protein conc...
Embodiment 2
[0146] Embodiment 2: Using transgenic rabbit milk as raw material
[0147] (1) Pretreatment of transgenic rabbit milk
[0148] The transgenic rabbit milk was collected, and the isoelectric points of casein and HuBChE were different, and the phosphate buffer solution of 1 M pH6.0 was used for freezing treatment to remove the casein.
[0149] Take the processing of 40ml milk sample as an example:
[0150] A. Take 40ml of rabbit milk, centrifuge at 10000g for 10min at 4°C, and remove the fat in the upper layer.
[0151] B. Measure the volume of the remaining milk sample, and add 1M pH6.0 phosphate buffer to a final concentration of 0.2M.
[0152] C. Freezing and thawing the sample, and then centrifuging the completely thawed sample at 10,000 g for 10 min at 4°C to collect the supernatant.
[0153] 6. After the supernatant was concentrated, the solution was replaced with an equal amount of buffer E (25mmol / L PB, 1mmol / L EDTA, 140mol / LNaCl, pH7.2).
[0154] (2) Affinity chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com