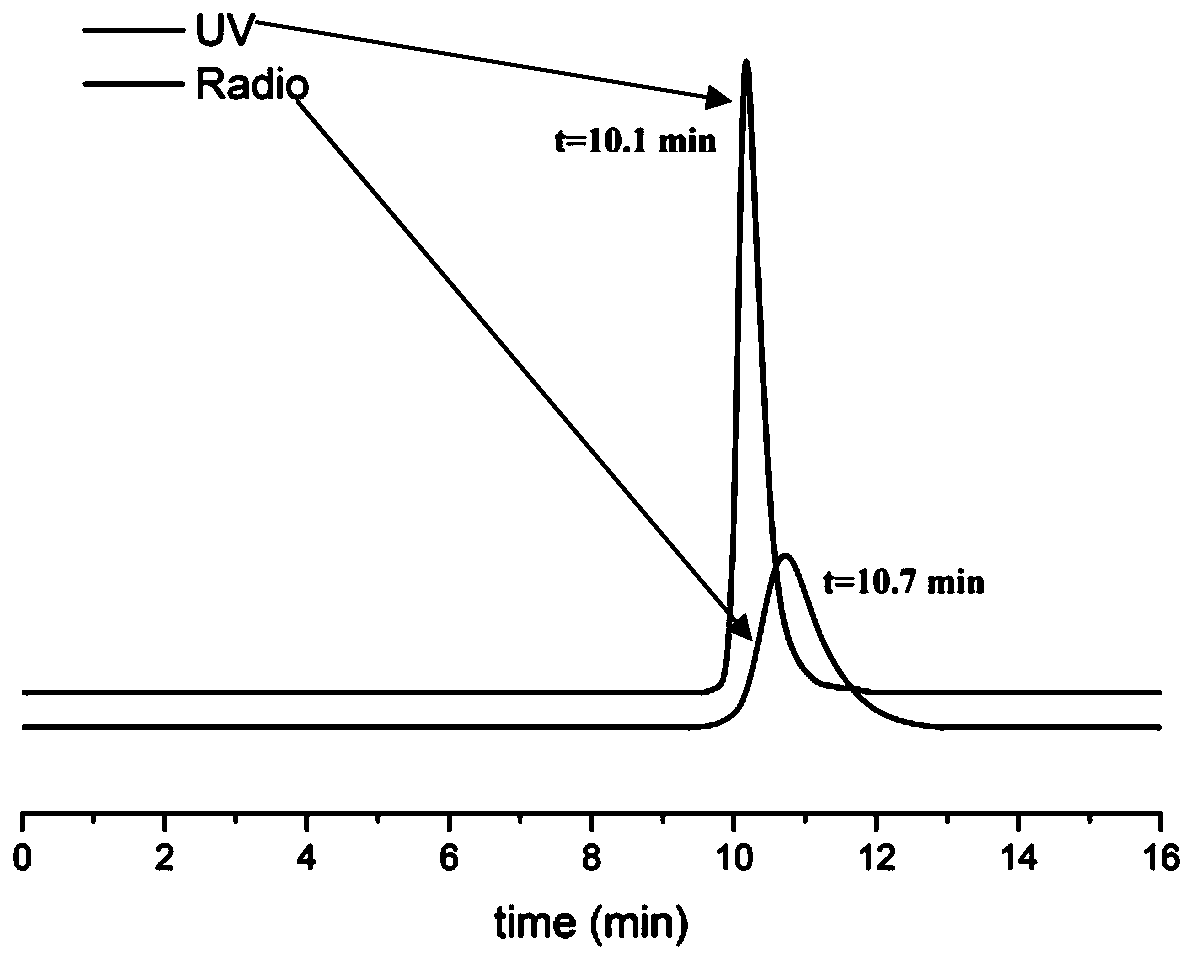
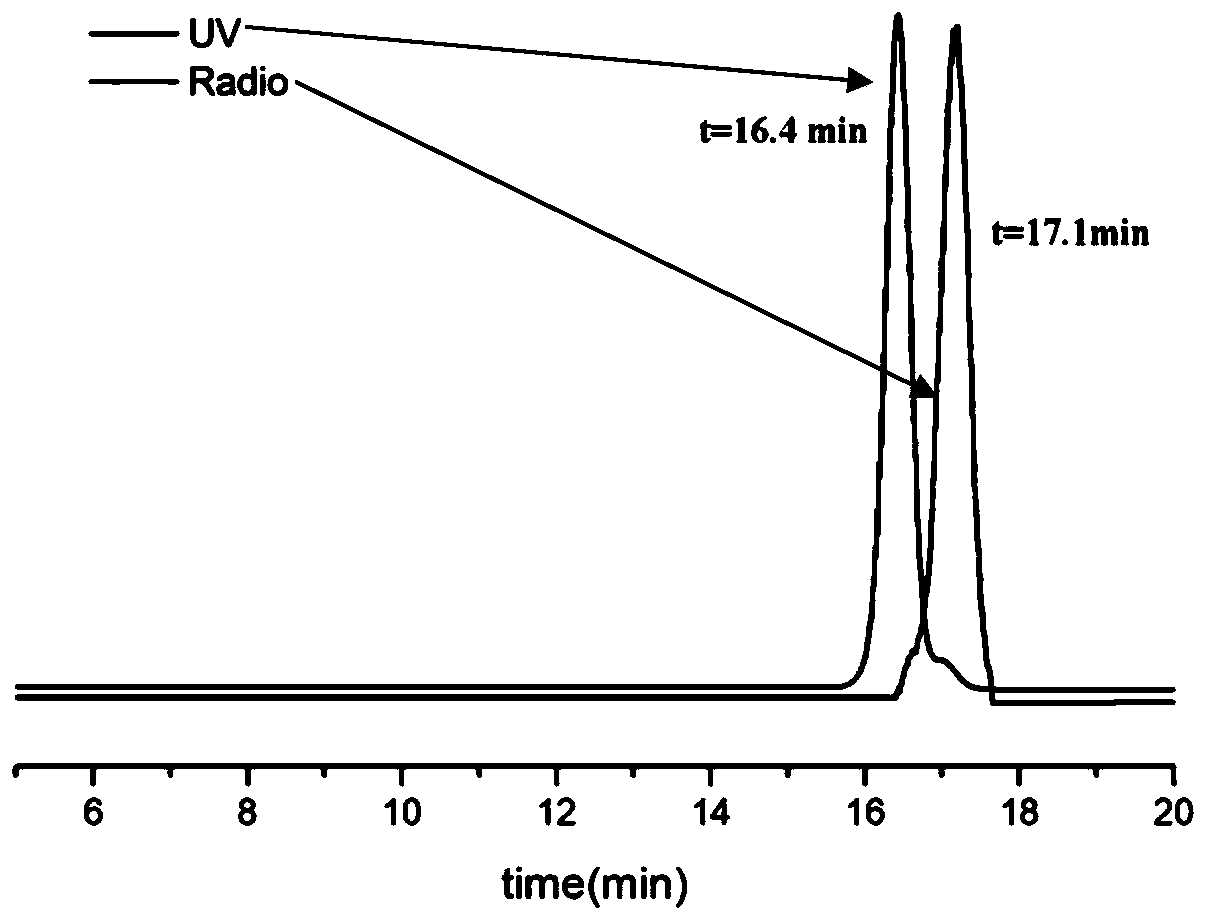
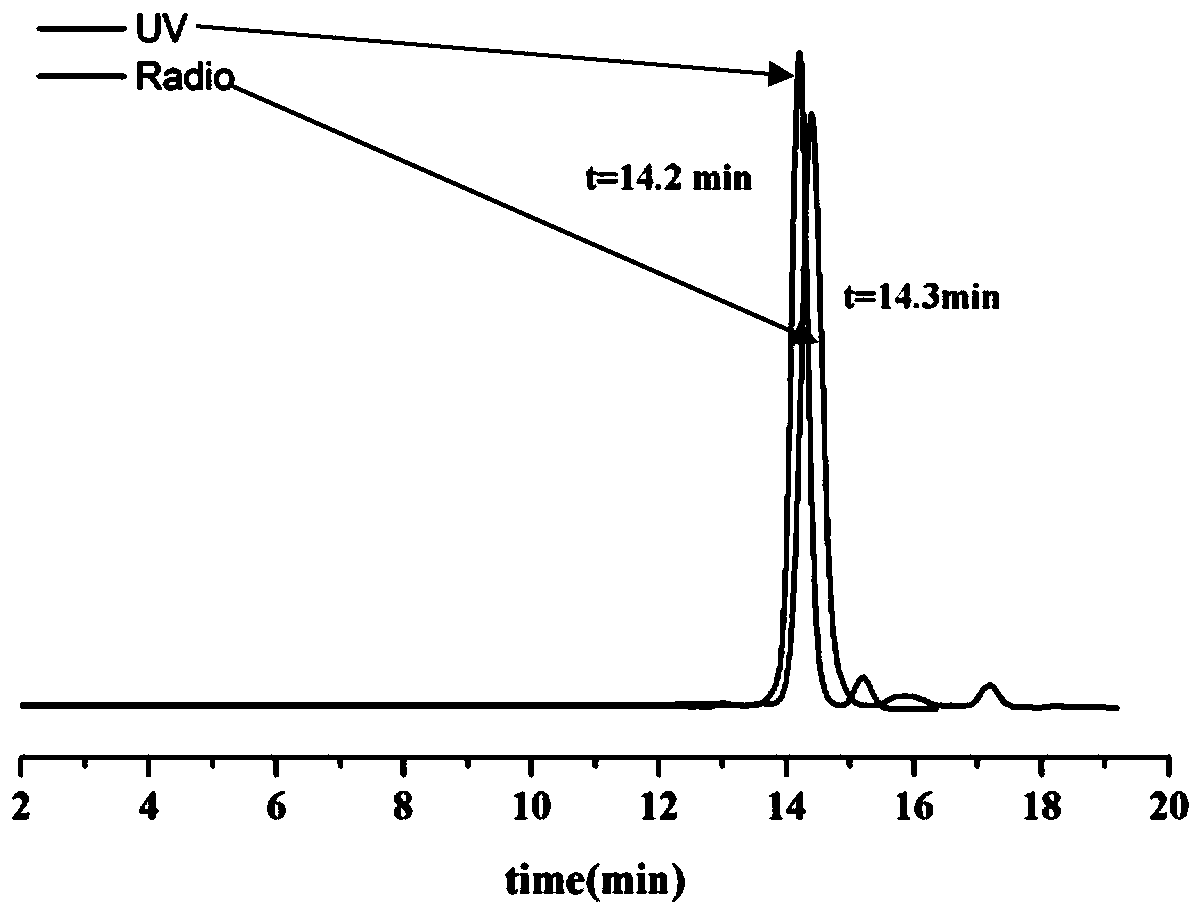
FAK targeting compounds, markers thereof, and preparation methods and applications thereof
A technology of compounds and precursor compounds, applied in the field of compounds, can solve the problem that the tumor suppressing activity needs to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Embodiment 1. The organic synthesis of compound 27aa
[0144] The DMF (300mL) solution of compound 2 (20g, 183.4mmol) was cooled to 0°C under an ice bath, sodium hydride (8.8g, 220mmol, 60% in mineral oil, 1.2 equiv) was added in batches, stirred at 0°C for 1h, 2-Chloro-3-nitropyridine (compound 1) (20 g, 126.5 mmol, 0.7 equiv) was added, slowly raised to room temperature, and stirring continued for 5 h. After the reaction, DMF was poured into 1L of water, and then extracted with ethyl acetate (300 mL*3 times). The ethyl acetate layers were combined, dried over anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, and separated by silica gel column chromatography (petroleum ether / ethyl acetate=10 / 1 to 3 / 1) to obtain compound 3 (22.2g, brown-yellow solid , yield 75.9%); The structure of compound 3 is characterized as follows:
[0145] 1 H NMR (400MHz, CDCl 3 ,δppm):8.67(dd,J=1.6Hz,4.7Hz,1H),8.26-8.23(m,1H),7.47-7.44(m,1H),3.42(s,3H),3.07(s,3H) .
...
Embodiment 2
[0168] Embodiment 2. The organic synthesis of compound 27ab
[0169] Compound 4 was obtained by referring to the method of Example 1, and 5-bromo-2,4-dichloropyrimidine was used instead of 2,4,5-trichloropyrimidine to react with compound 4 to obtain compound 6b having a structure similar to compound 6a;
[0170] Compound 12 was obtained by referring to the method of Example 1, and compound 6b was used instead of compound 6a to react with compound 12 to obtain compound 27ab (0.51 g, light green solid, yield 33.1%) having a structure similar to compound 27aa. The structure of compound 27ab is characterized as follows:
[0171] 1 H NMR (400MHz, CDCl 3 ,δppm):8.80(d,J=6.3Hz,1H),8.35(s,1H),8.19-8.17(m,2H),7.94(d,J=5.8Hz,1H),7.29-7.27(m, 1H), 7.19(s, 1H), 6.53(d, J=1.6Hz, 1H), 6.47(dd, J=1.6Hz, 5.8Hz, 1H), 3.85(s, 3H), 3.69(t, J= 3.4Hz, 2H), 3.29(s, 3H), 3.20(t, J=3.1Hz, 4H), 3.05(s, 3H), 2.75(t, J=2.8Hz, 4H), 2.66(t, J= 3.4Hz, 2H).
[0172] 13 C NMR (400MHz, CDCl 3 ,δppm):1...
Embodiment 3
[0174] Embodiment 3. The organic synthesis of compound 27ac
[0175] Compound 4 was prepared by referring to the method of Example 1, and 5-fluoro-2,4-dichloropyrimidine was used instead of 2,4,5-trichloropyrimidine to react with compound 4 to obtain compound 6c having a structure similar to compound 6a.
[0176] Referring to the method of Example 2, compound 6c was used instead of compound 6b to react with compound 12 to obtain compound 27ca (0.98 g, yellow solid, yield 59.6%) which was similar in structure to compound 27ab. The structure of compound 27ac is characterized as follows:
[0177] 1 H NMR (400MHz, CDCl 3 ,δppm):8.85(d,J=8.2Hz,1H),8.19(dd,J=1.2Hz,4.4Hz,1H),8.00-7.96(m,3H),7.32-7.29(m,1H),7.19 (s,1H),6.54(d,J=2.2Hz,1H),6.50(dd,J=2.0Hz,8.7Hz,1H),3.86(s,3H),3.75(t,J=4.8Hz,2H ),3.28(s,3H),3.23(s,4H),3.04(s,3H),2.82(s,4H),2.72(s,2H).
[0178] 13 C NMR (400MHz, CDCl 3 ,δppm):155.35,149.20,146.75,143.76,142.29,142.00,141.16,140.97,132.96,130.34,123.76,122.44,120.18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com