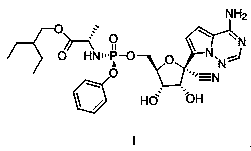
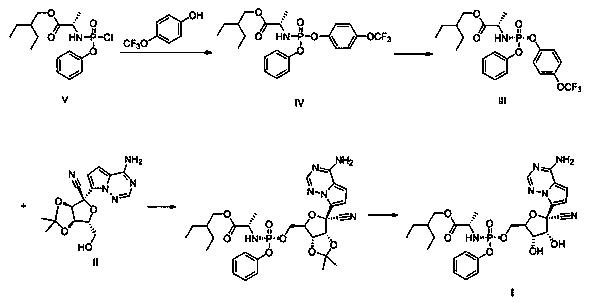
Preparation method of retegravir
A compound, trifluoromethoxyphenol technology, applied in the field of preparation of remdesivir, can solve the problems of increasing the risk of genotoxic impurities, and achieve the effect of reducing the risk of genotoxic impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of compound IV:
[0028] Under the protection of nitrogen, add dichloromethane (100mL), compound V (16.48g, 47.4mmol) and 4-trifluoromethoxyphenol (8.01g, 45.0mmol) into the round bottom flask, cool down to 0°C, and slowly Slowly drop dichloromethane solution (30mL) containing triethylamine (6.28mL), keep the temperature of the solution in the round bottom flask not higher than 5°C, after the dropwise addition, slowly rise to room temperature (15~20°C), And stir at room temperature for 24 hours; add cold water (100g of ice + water in total, cool down the system to 0~5°C, note that ice is added first to cool down, and the final temperature is below 5°C, but no solid ice exists. Other examples are the same), After stirring, the layers were separated, and the organic layer was washed with saturated brine, and then washed with 10gMg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to d...
Embodiment 2
[0036] The preparation of compound IV:
[0037] Under the protection of nitrogen, add compound V (15.62g, 45mmol), 4-trifluoromethoxyphenol (8.01g, 45.0mmol), dichloromethane (100mL) into a round bottom flask, cool down to 5°C, and Ethylamine (6.28mL) in dichloromethane solution (30mL) was slowly added dropwise to the reaction system, keeping the temperature of the solution in the round bottom flask below 10°C, after the dropwise addition was completed, slowly rise to room temperature (15~20°C) , and stirred at room temperature for 12 hours; add cold water (100g of ice + water, the system cools down to 0~5°C), cool the system down to 5°C, stir and separate layers, wash the organic layer with saturated brine, wash with 10gMg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to dryness under reduced pressure to obtain 21.1 g of crude compound IV, with a molar yield of 95.8% (0.958 moles of crude compound IV ...
Embodiment 3
[0044] The preparation of compound IV:
[0045] Under nitrogen protection, compound V (18.74g, 54mmol), 4-trifluoromethoxyphenol (8.01g, 45.0mmol), dichloromethane (100mL) were added to a round bottom flask, cooled to 5°C, and then slowly Add dropwise a dichloromethane solution (30mL) containing triethylamine (6.28mL), keep the temperature of the solution in the round bottom flask below 10°C, after the dropwise addition, slowly rise to room temperature (15~20°C), and Stir at room temperature for 16 hours; add cold water (100g of ice + water in total, cool down the system to 0~5°C), stir and separate layers, wash the organic layer with saturated brine, wash with 10g Mg 2 SO 4 Stir dry. After filtration, the organic layer was concentrated, then 100 mL of toluene was added, and evaporated to dryness under reduced pressure to obtain 21.2 g of crude compound IV, with a molar yield of 96.3% (0.963 moles of crude compound IV per mole of 4-trifluoromethoxyphenol), without further pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com