Method for preparing synthesis gas by methane chemical-looping partial oxidation
A chemical chain and synthesis gas technology, applied in chemical instruments and methods, inorganic chemistry, iron compounds, etc., can solve the problems of iron-based oxygen carrier selectivity and carbon deposition resistance, and achieve excellent synthesis gas selectivity and oxygen carrying capacity, excellent methane activation performance, suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
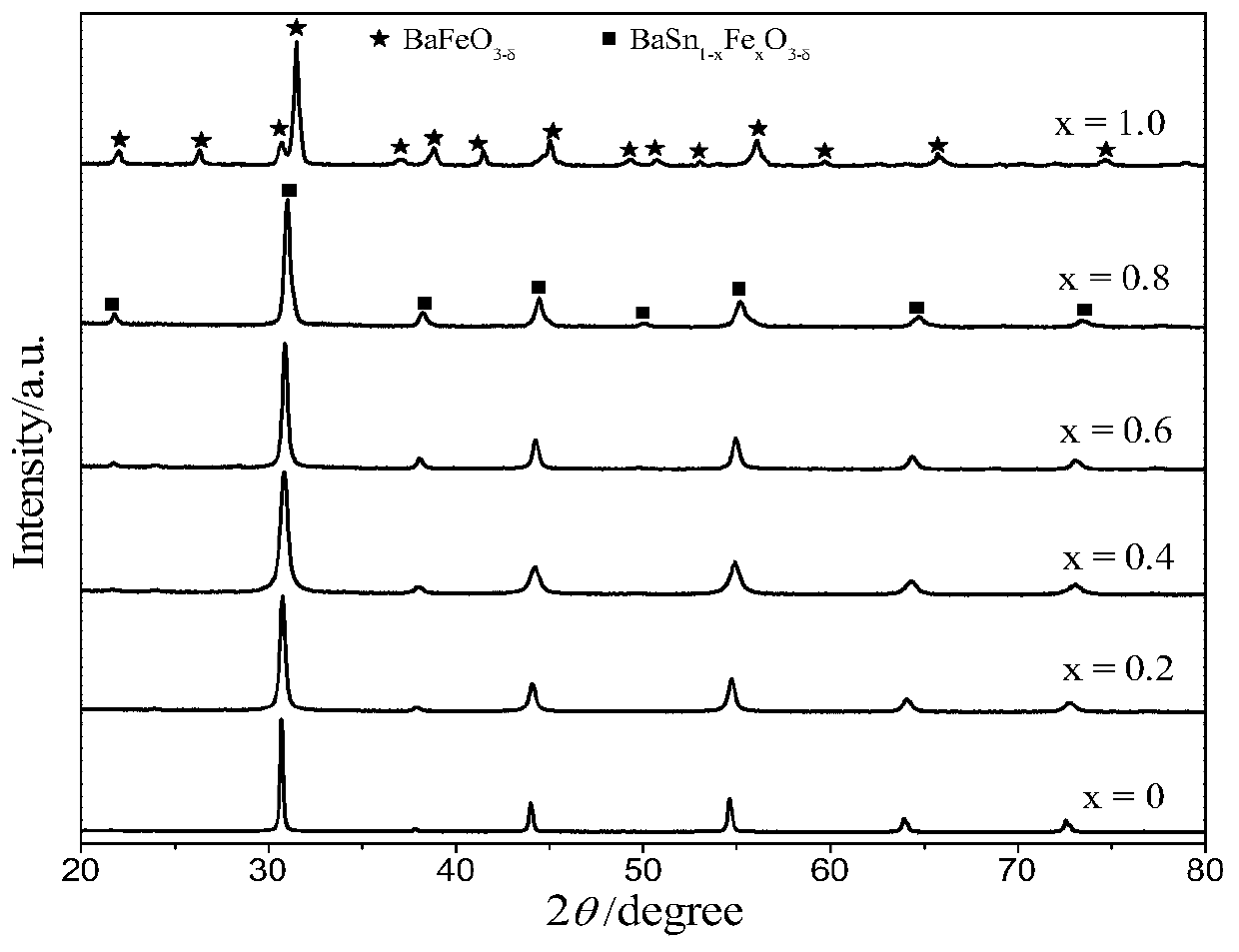
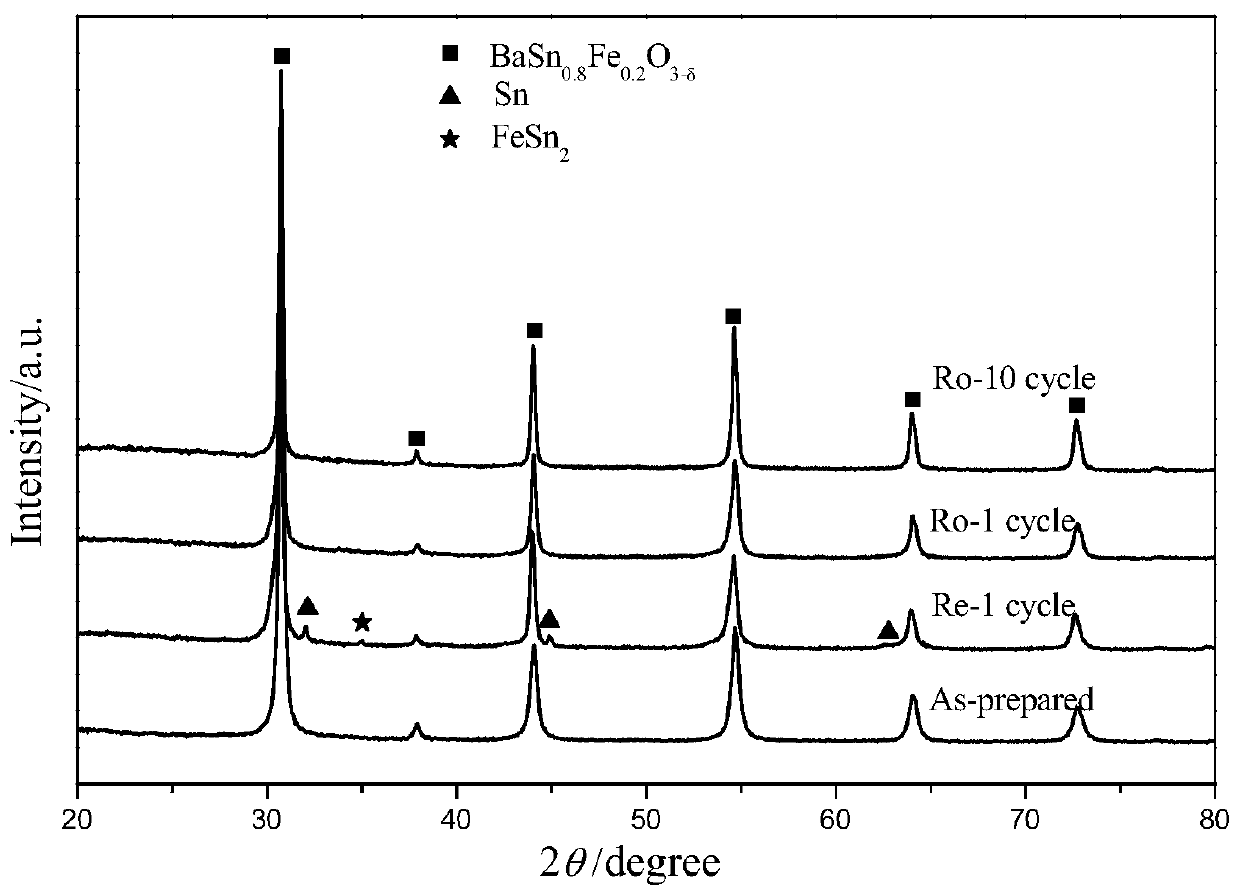
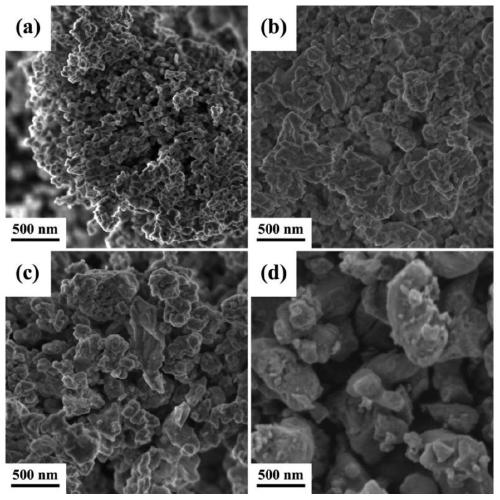
[0060] BaSn 0.8 Fe 0.2 o 3-δ Preparation of oxygen carrier
[0061] SnC 2 o 4 Mix with hydrogen peroxide / water solution, fully stir until dissolved, add a certain amount of citric acid and stir to dissolve, the ratio of citric acid to the total amount of Sn ions is 6 / 1, then drop ammonia water to adjust the pH to about 7, and obtain Tin mother liquor; a certain stoichiometric ratio of Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 0 mix and add deionized water, add citric acid after stirring and dissolving, the ratio of citric acid to the total amount of Ba and Fe cations is 6 / 1, after dissolving, adjust the pH to 5-8 with ammonia water after dissolving; Mix the above two solutions, put them in a water bath at 80°C until a gel is formed, dry in an oven at 150°C overnight to obtain a black precursor, grind the precursor, slowly raise the temperature to 450°C in a muffle furnace, and roast for 4 hours, then grind again Afterwards, bake at 900°C for 4 hours in a high-temperature furn...
Embodiment 2
[0063] BaSn 0.6 Fe 0.4 o 3-δ Preparation of oxygen carrier
[0064] The specific operation is the same as above, the difference is that the amount of tin-containing iron mother liquor added is changed, and the amount of deionized water added is changed at the same time to obtain oxygen carriers with different Sn doping amounts.
Embodiment 3
[0066] BaSn 0.4 Fe 06 o 3-δ Preparation of oxygen carrier
[0067] The specific operation is the same as above, the difference is that the amount of tin-containing iron mother liquor added is changed, and the amount of deionized water added is changed at the same time to obtain oxygen carriers with different Sn doping amounts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com