F3 polypeptide-targeted doxorubicin prodrug with pH sensitivity and preparation method thereof
A doxorubicin and sensitivity technology, applied in the field of doxorubicin prodrug and its preparation, can solve the problems of low selectivity of doxorubicin, prominent toxic and side effects, etc., and achieve the effect of reducing toxic and side effects and avoiding release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
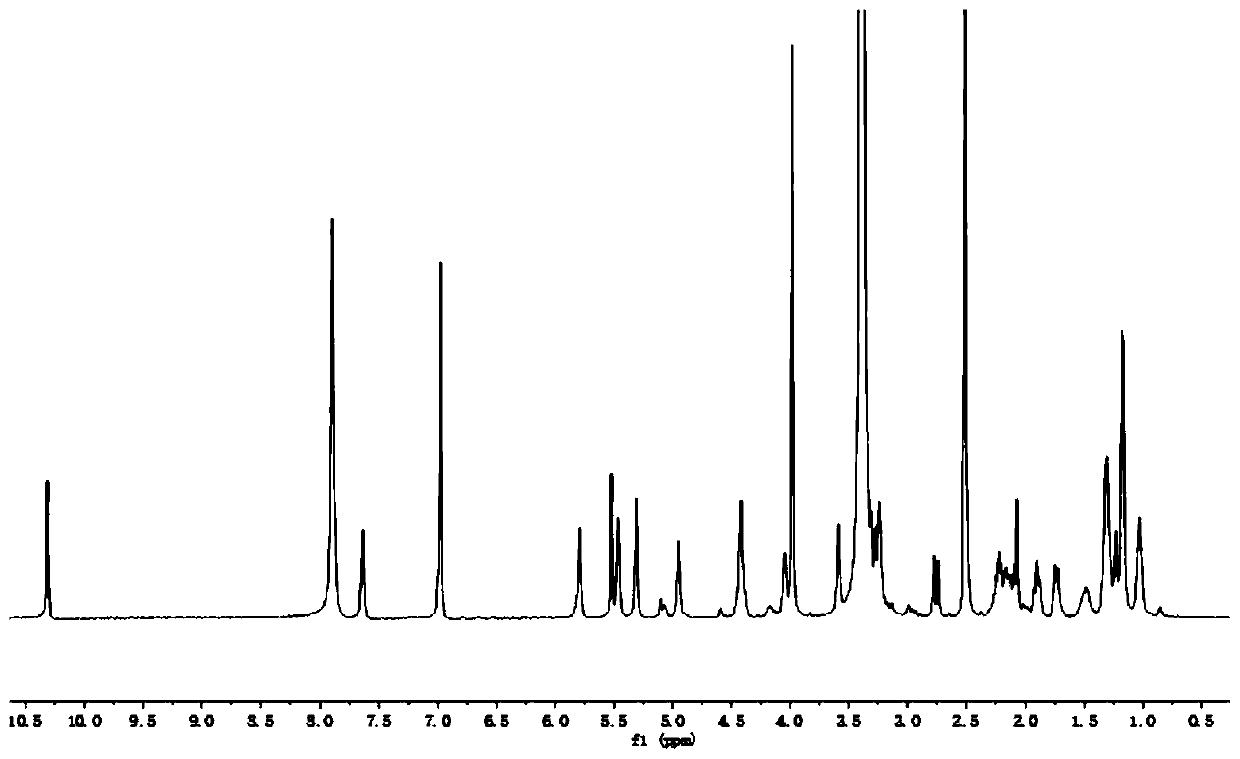
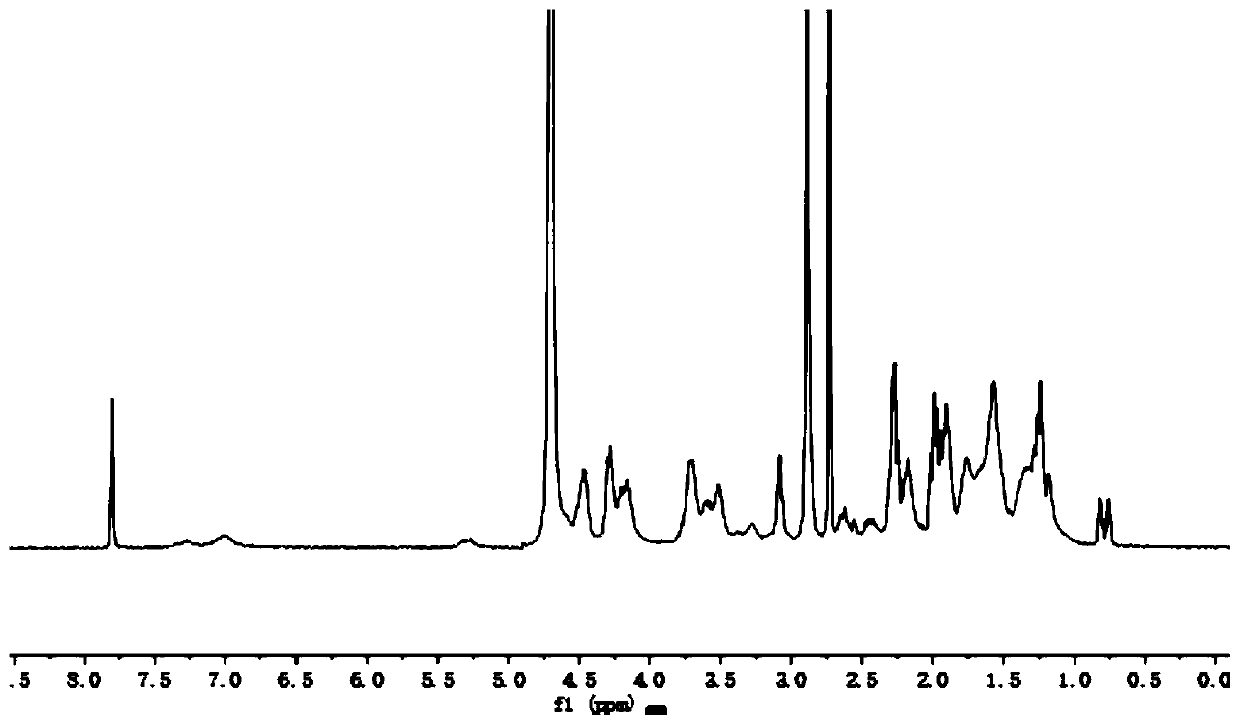
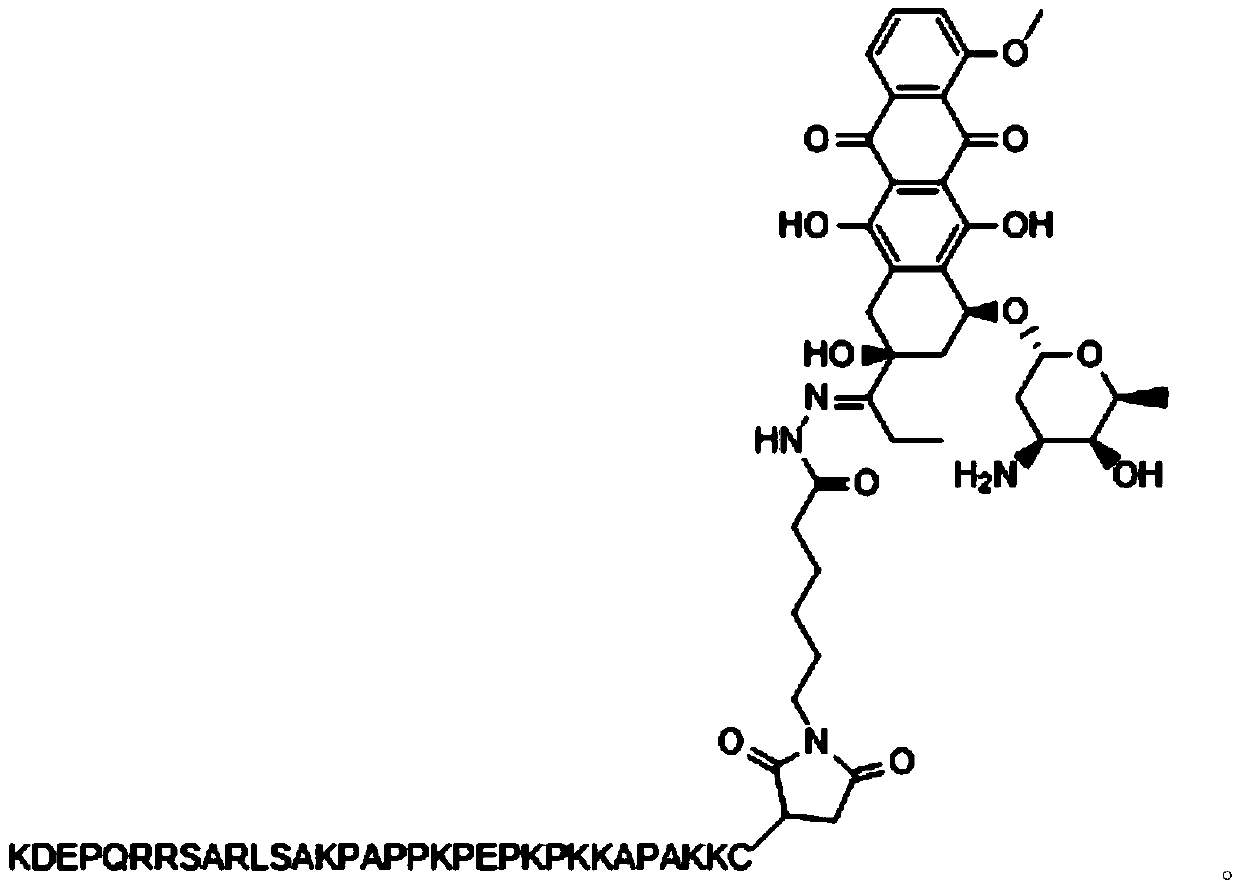
[0030] A F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug, composed of 6-maleimidocaproic acid hydrazide and doxorubicin linked by a pH-sensitive acylhydrazone bond, and then combined with the The mercapto group is prepared by Michael addition reaction, wherein the 6-maleimidocaproic acid hydrazide is prepared by hydrazide reaction of 6-maleimidocaproic acid.
[0031] The above-mentioned F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug is specifically prepared by the following method:
[0032] 1) Preparation of 6-maleimide caproic acid hydrazide: Dissolve 400 mg of 6-maleimide caproic acid in anhydrous tetrahydrofuran, cool to 4°C, add 208 μL N-methylmorpholine, 257mg of isobutyl chloroformate, 250mg of tert-butyl carbazate was added after 5 minutes, and kept at 4°C for 30 minutes, then stirred at room temperature for 1 hour to carry out hydrazide reaction, after the hydrazide reaction was completed, filter, The solvent was evaporated, the residue was ...
Embodiment 2
[0041] A F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug, composed of 6-maleimidocaproic acid hydrazide and doxorubicin linked by a pH-sensitive acylhydrazone bond, and then combined with the The mercapto group is prepared by Michael addition reaction, wherein the 6-maleimidocaproic acid hydrazide is prepared by hydrazide reaction of 6-maleimidocaproic acid.
[0042] The above-mentioned F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug is specifically prepared by the following method:
[0043] 1) Preparation of 6-maleimide caproic acid hydrazide: Dissolve 400mg of 6-maleimide caproic acid in anhydrous tetrahydrofuran, cool to 4°C, add 270 μL N-methylmorpholine, 334mg of isobutyl chloroformate, after 5-10min, add 325mg of tert-butyl carbazate, and keep at 4°C for 40min, then, stir at room temperature for 1.5h, carry out hydrazide reaction, after the hydrazide reaction is completed , filtered, evaporated the solvent, and dissolved the residue in ethyl ac...
Embodiment 3
[0048] A F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug, composed of 6-maleimidocaproic acid hydrazide and doxorubicin linked by a pH-sensitive acylhydrazone bond, and then combined with the The mercapto group is prepared by Michael addition reaction, wherein the 6-maleimidocaproic acid hydrazide is prepared by hydrazide reaction of 6-maleimidocaproic acid.
[0049] The above-mentioned F3 polypeptide-targeted and pH-sensitive doxorubicin prodrug is specifically prepared by the following method:
[0050] 1) Preparation of 6-maleimide caproic acid hydrazide: Dissolve 400 mg of 6-maleimide caproic acid in anhydrous tetrahydrofuran, cool to 4°C, add 312 μL N-methylmorpholine, 386mg of isobutyl chloroformate, 375mg of tert-butyl carbazate was added after 5-10min, and kept at 4°C for 50min, and then stirred at room temperature for 2h to perform hydrazide reaction. After the hydrazide reaction was completed, Filter, evaporate the solvent, and dissolve the residue in et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com