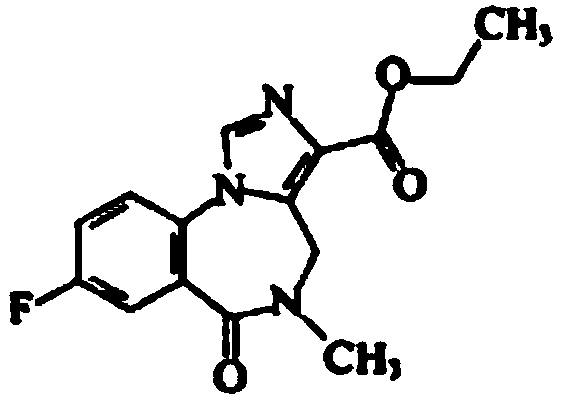
Flumazenil inclusion compound and production method and application thereof
A technology of flumazenil and clathrate, applied in the field of flumazenil clathrate and its preparation, can solve the problems of low bioavailability, limited solubility of flumazenil, etc., achieve improved solubility and simple preparation conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
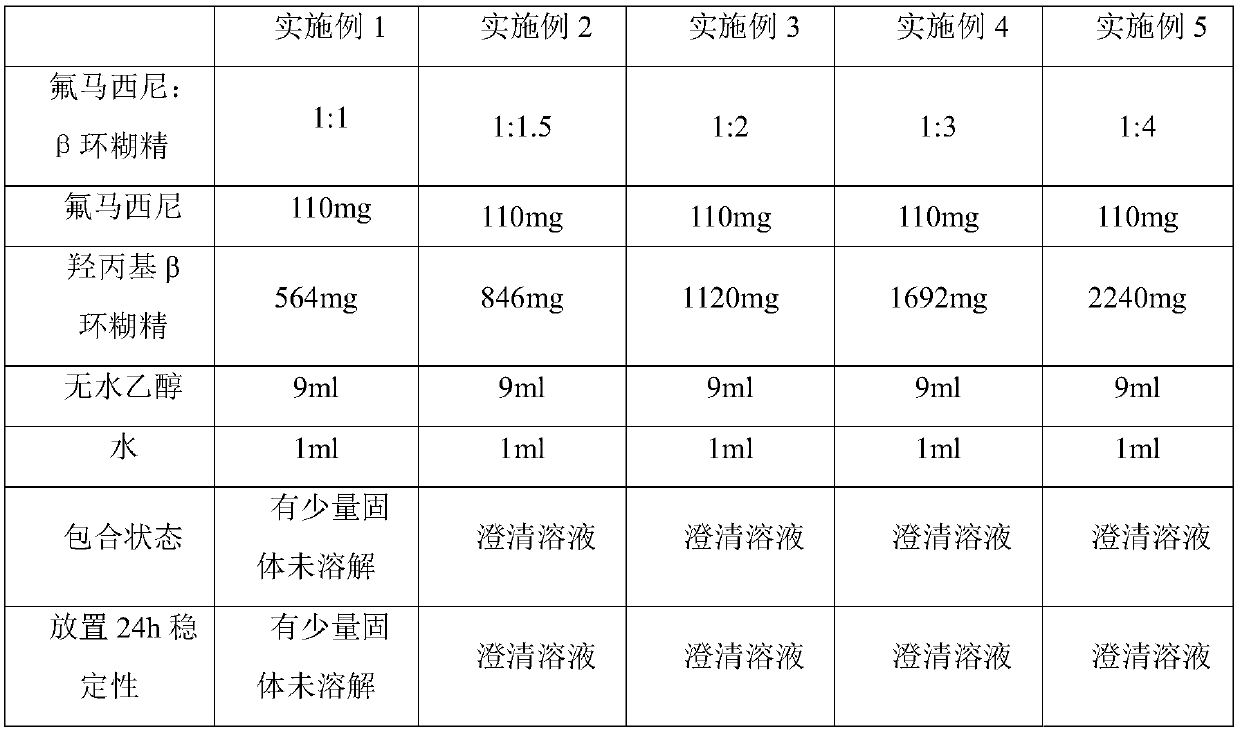
[0031] Embodiments 1-5 relate to the preparation of a flumazenil β-cyclodextrin inclusion compound. The specific components in each embodiment are shown in Table 1 below, and the preparation method is as follows:
[0032] Weigh 110 mg of flumazenil, weigh corresponding hydroxypropyl β-cyclodextrin according to Example 1-5 and place it in a round-bottomed flask, then add 9 ml of absolute ethanol and 1 ml of water to the round-bottomed flask. The inclusion of flumazenil and β-cyclodextrin was carried out on a constant temperature magnetic stirrer at 45° C. and a rotational speed of 200 r / min, and the inclusion time was 30 minutes.
[0033] Table 1. Inclusion of flumazenil and hydroxypropyl β-cyclodextrin in different molar ratios
[0034]
[0035] As can be seen from the above Table 1, when the molar ratio of flumazenil to hydroxypropyl β cyclodextrin is 1:1, the inclusion compound of flumazenil β cyclodextrin cannot be completely prepared, and the With the increase of the c...
Embodiment 6-15
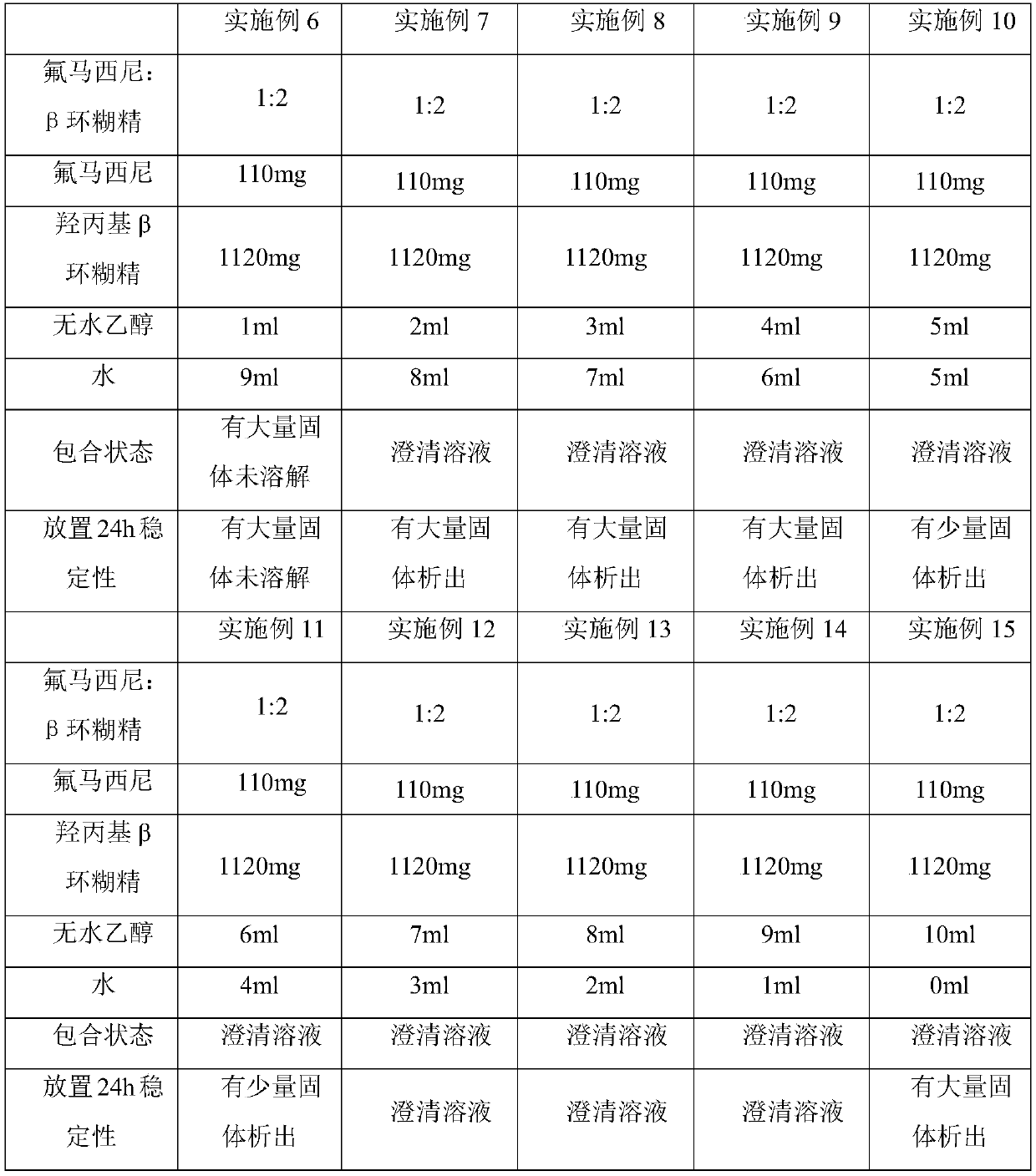
[0037] Examples 6-15 relate to the preparation of a flumazenil β-cyclodextrin inclusion compound. The specific components in each example are shown in Table 2 below, and the preparation method is as follows:
[0038] Weigh 110 mg of flumazenil and 1120 mg of hydroxypropyl β-cyclodextrin in a round-bottomed flask, then add corresponding amounts of absolute ethanol and water to the round-bottomed flask according to Examples 6-15, and stir magnetically at a constant temperature The inclusion of flumazenil and β-cyclodextrin was carried out on the machine at 45°C and the rotation speed was 200r / min, and the inclusion time was 30min.
[0039] Table 2. Inclusion of mixed solvents with different ratios of ethanol and water
[0040]
[0041] As can be seen from the above table 2, when the volume fraction of the ethanol solution is 10%, the inclusion compound of flumazenil β-cyclodextrin cannot be completely prepared; as the volume fraction of the ethanol solution increases, the vol...
Embodiment 16-19
[0043] Examples 16-19 relate to the preparation of a flumazenil β-cyclodextrin inclusion compound. The specific components in each example are shown in Table 3 below, and the preparation method is as follows:
[0044] Weigh 110 mg of flumazenil and 1120 mg of hydroxypropyl β-cyclodextrin in a round-bottomed flask, then add 9 ml of absolute ethanol and 1 ml of water to the round-bottomed flask. -19 The inclusion of flumazenil and β-cyclodextrin was carried out at a certain temperature and the rotation speed was 200r / min, and the inclusion time was 30min.
[0045] Table 3. Inclusion of flumazenil and hydroxypropyl β-cyclodextrin at different temperatures
[0046]
[0047] It can be known from the above Table 3 that when the inclusion temperature of flumazenil and hydroxypropyl β-cyclodextrin is 30°C, the inclusion is complete, and the inclusion compound of flumazenil β-cyclodextrin can be prepared. The increase of β-cyclodextrin can still be completely included, so the selec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com