Method for synthesizing 2,2,4-trimethyl-1,3-pentanediolmono(2-methylpropanoate) by adopting micro-channel reactor
A technology of pentanediol monoisobutyrate and microchannel reactor, which is applied in chemical instruments and methods, chemical/physical/physicochemical reactors, carboxylate preparation, etc., to achieve precise controllable temperature and precise reaction conditions Controllable, great results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
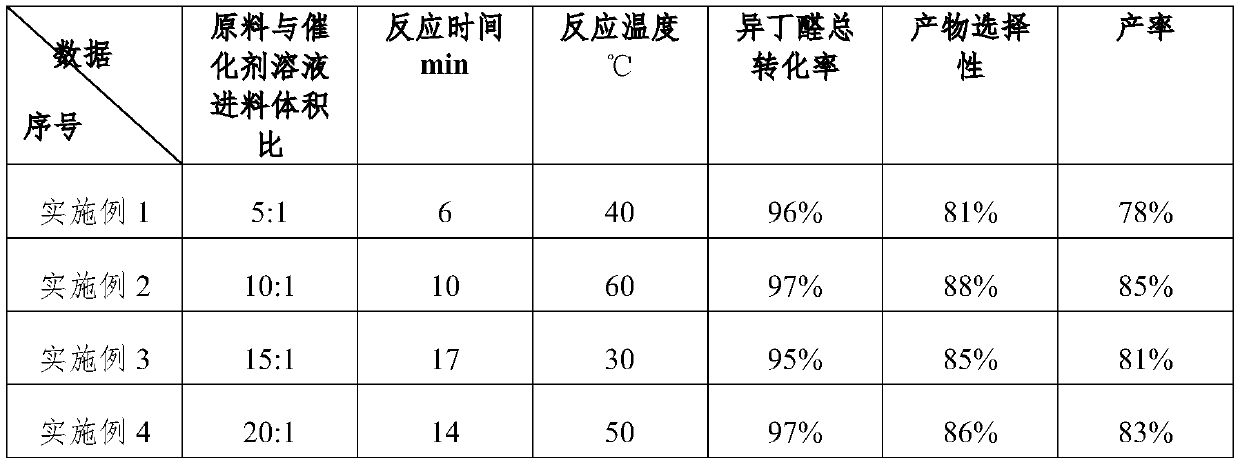
Embodiment 1
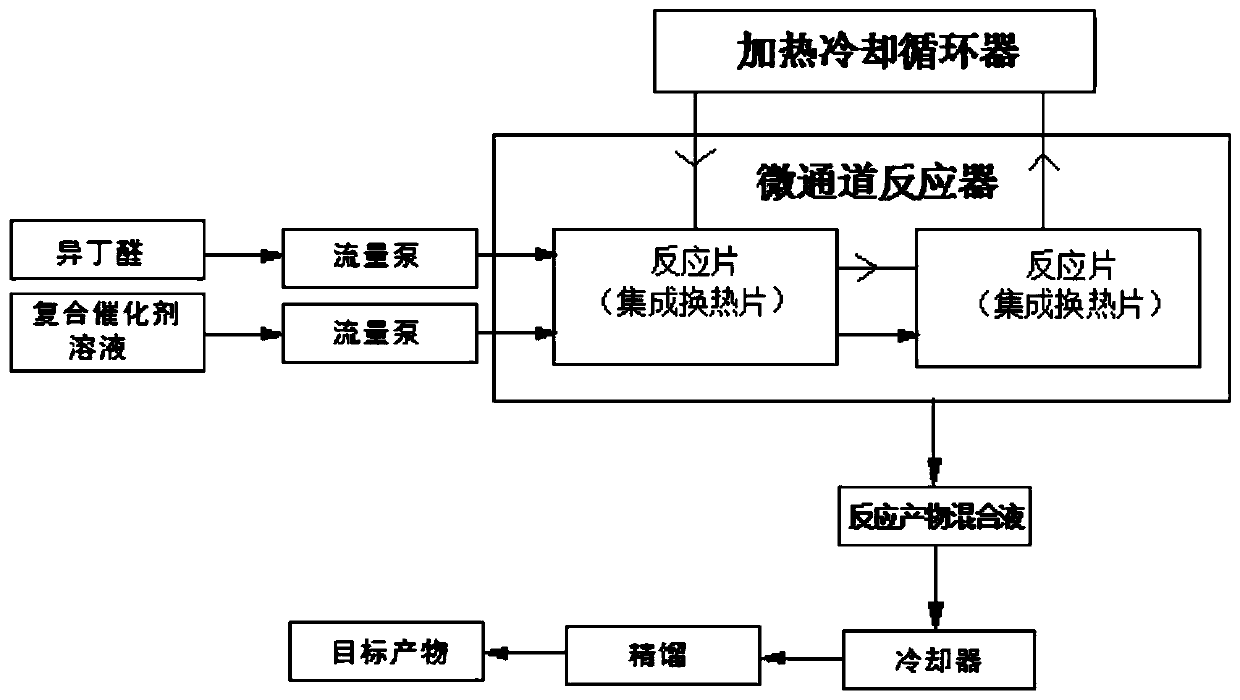
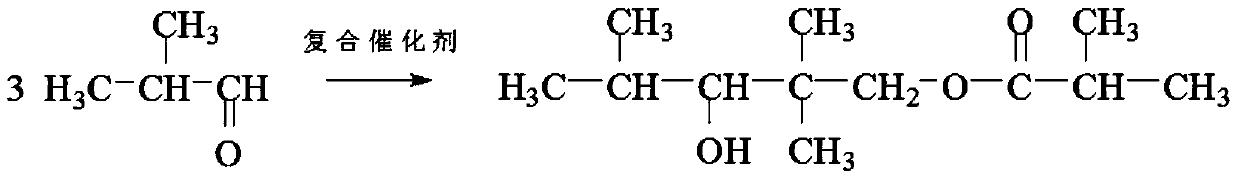
[0027] The method for synthesizing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate using a microchannel reactor of the present invention, using isobutyraldehyde as a raw material, and brominating with sodium hydroxide and tetrabutyl The composite solution of ammonium configuration is a composite catalyst solution, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate is synthesized in a microchannel reactor (see attached figure 1 ), including the following steps:
[0028] S1: Configuration of the composite catalyst solution, dissolving sodium hydroxide and tetrabutylammonium bromide in deionized water to form a composite catalyst solution of a certain concentration, wherein the mass concentration of sodium hydroxide is 20%, tetrabutylammonium bromide The mass concentration is 0.6%;
[0029] S2: The setting of the reaction conditions of the microchannel reactor, the heat exchange sheet integrated with the reaction sheet in the microchannel reactor is connected to the external he...
Embodiment 2
[0033]The method for synthesizing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate using a microchannel reactor of the present invention, using isobutyraldehyde as a raw material, and brominating with potassium hydroxide and tetrabutyl The composite solution configured by ammonium is a composite catalyst solution, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate is synthesized in a microchannel reactor, including the following steps:
[0034] S1: Configuration of the composite catalyst solution, dissolving potassium hydroxide and tetrabutylammonium bromide in deionized water to form a composite catalyst solution of a certain concentration, wherein the mass concentration of potassium hydroxide is 30%, tetrabutylammonium bromide The mass concentration is 1%;
[0035] S2: The setting of the reaction conditions of the microchannel reactor, the heat exchange sheet integrated with the reaction sheet in the microchannel reactor is connected to the external heating and cooling circu...
Embodiment 3
[0039] The method for synthesizing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate using a microchannel reactor of the present invention, using isobutyraldehyde as a raw material, and brominating with potassium hydroxide and tetrabutyl The composite solution configured by ammonium is a composite catalyst solution, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate is synthesized in a microchannel reactor, including the following steps:
[0040] S1: Configuration of the composite catalyst solution, dissolving potassium hydroxide and tetrabutylammonium bromide in deionized water to form a composite catalyst solution of a certain concentration, wherein the mass concentration of potassium hydroxide is 10%, tetrabutylammonium bromide The mass concentration is 0.8%;
[0041] S2: The setting of the reaction conditions of the microchannel reactor, the heat exchange sheet integrated with the reaction sheet in the microchannel reactor is connected to the external heating and cooling ci...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap