Biomarker for gastric cancer diagnosis and prognosis evaluation, application of biomarker and detection kit
A biomarker and prognosis assessment technology, applied in the field of biomedicine, can solve problems such as passive and unfavorable patient condition control, achieve high clinical application value, facilitate the prediction of gastric cancer prognosis, and save medical costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
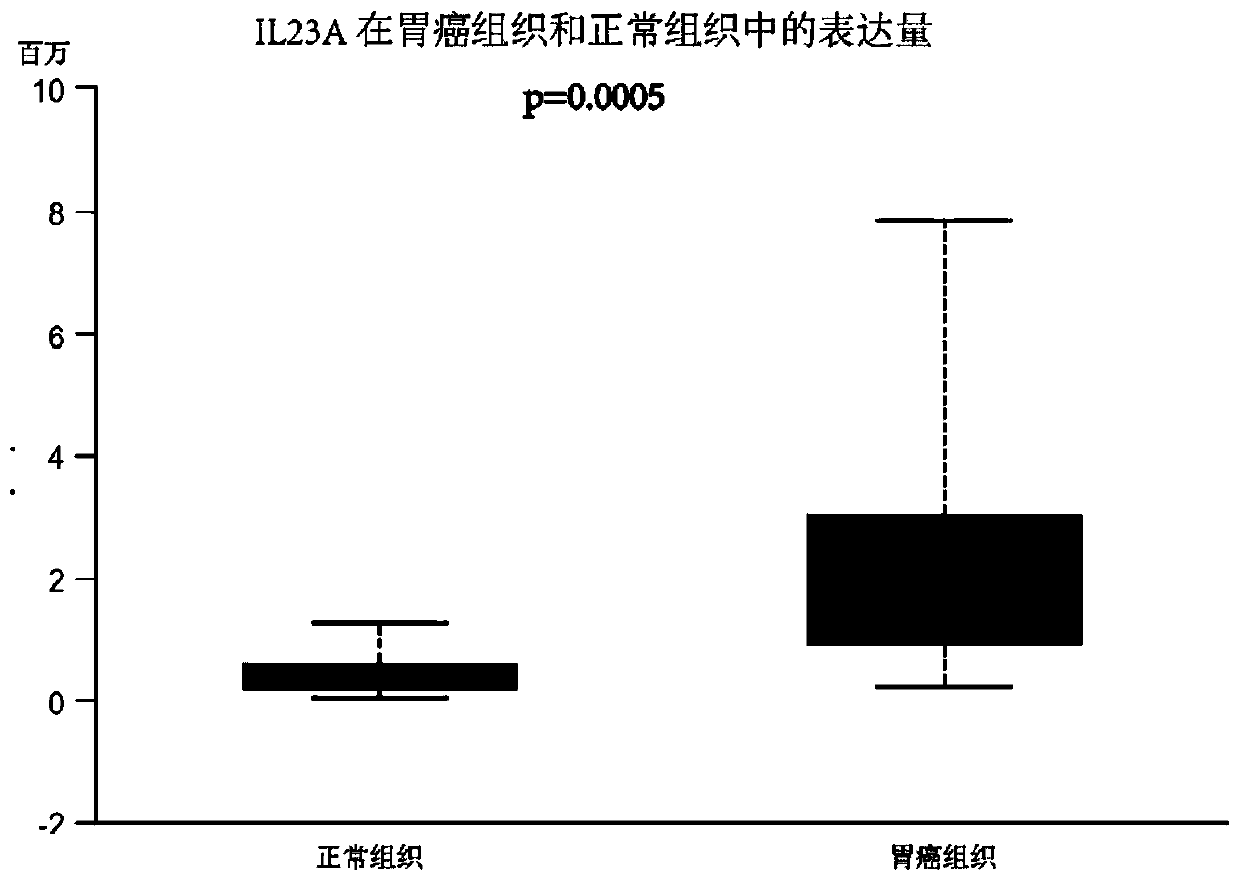
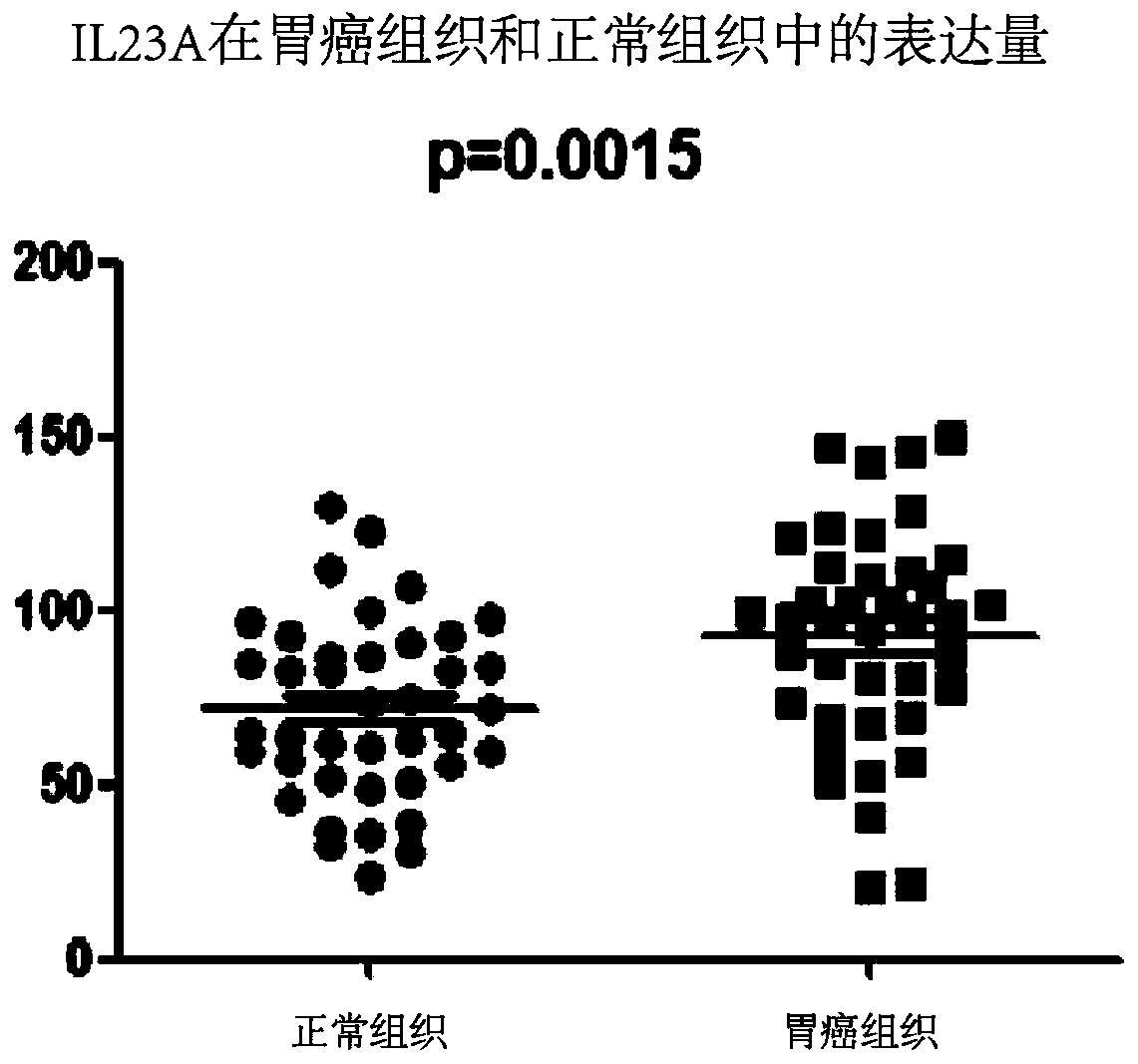
[0037] Example 1: Detection of expression levels in collected human gastric cancer tissues and adjacent normal tissues by qPCR
[0038] 1. RNA extraction
[0039] 1) Grinding of gastric cancer tissue and paracancerous tissue
[0040] Clean the mortar, pestle, scissors, scalpel, tweezers, and spoons required for grinding with ultrapure water, bake in an oven at 60°C for 3 hours, wrap them in foil paper, and bake in an oven at 180°C overnight; Before tissue grinding, the above-mentioned equipment needs to be pre-cooled by liquid nitrogen; take out the clinical samples stored in liquid nitrogen, cut out soybean-sized tissue pieces and put them in a pre-cooled mortar for grinding, and at the same time, replenish them in small amounts and multiple times. Liquid nitrogen, keep the mortar at ultra-low temperature until it is ground into powder, this process generally takes 10min-15min; after the grinding is complete, add 1mL pre-cooled TRIzoI to continue grinding, and mix the tissue...
Embodiment 2
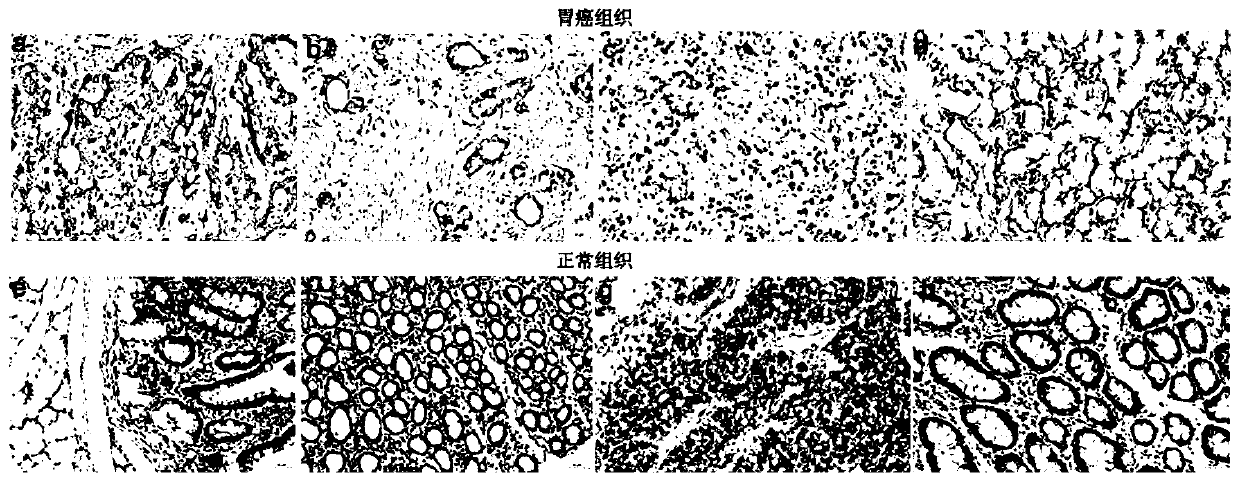
[0073] Example 2. Tissue microarray immunohistochemical detection of expression levels in gastric cancer tissues and adjacent normal tissues:
[0074] 1. Frozen sections of gastric cancer tissue and paracancerous tissue
[0075] Mark the OCT embedding agent (opti-mum cutting temperature compound) on the fixed head, and then take a tissue of appropriate size (about 24×24×3mm, without embedding) and place it on the OCT. The surface of the tissue is exposed outside the OCT. All tissues were embedded with OCT. Put the fixed head on the quick-cooling table in the freezer, and freeze at -25°C for 10 minutes (the time varies slightly depending on the tissue, and the time for larger tissues or adipose tissue can be longer, generally about 10 minutes). Clamp the frozen head to the microtome, fix the angle of the high-speed knife, use fine adjustment to flatten the tissue to expose the maximum cut surface, adjust the fine adjustment scale to 5 μm, put down the anti-roll plate and start...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com