Drug delivery systems and methods
A catheter, main body technology, applied in the field of drug delivery systems and methods, can solve the problems of harmful, difficult to distribute, inefficient patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example A
[0194] Alternate pulsatile infusions of drug (pump 1) and buffer / saline (pump 2)
[0195] Total drug volume: 2.2mL
[0196] Total buffer volume: 4.4mL
[0197] Infusion rate with two pumps: 15mL / min
[0198] Circulation: 10 cycles in the lumbar area then 10 cycles in the cisterna magna
[0199] Time between loops: 100ms
[0200] Infusion Description: In the lumbar region, pump 1 infused 0.11 mL at 15 mL / min with a 100-ms pause, and pump 2 infused 0.22 mL at 15 mL / min with a 100-ms pause (cycle 1). This is repeated at the waist for a total of 10 cycles. The catheter is threaded upwards into the cisterna magna. Pump 1 infused 0.11 mL at 15 mL / min with a 100-ms pause, and pump 2 infused 0.22 mL at 15 mL / min with a 100-ms pause (cycle 1). This was repeated for a total of 10 cycles in the cisterna magna.
example B
[0202] Alternate pulsatile infusions of drug (pump 1) and buffer / saline (pump 2)
[0203] Total drug volume: 3mL
[0204] Total buffer volume: 20mL
[0205] Infusion rate of two pumps: 4mL / min
[0206] Cycles: 13 cycles in the chest area
[0207] Time between alternating pump 1 to pump 2: 1000 ms
[0208] Time between cycles (pump 2 to pump 1): 5000 milliseconds
[0209] Infusion Description: In the lumbar region, pump 1 infused 0.231 mL at 4 mL / min with a 1000 ms pause, pump 2 infused 2.0 mL at 4 mL / min with a 5000 ms pause (cycle 1). This is repeated for a total of 13 cycles in the chest area.
example C
[0211] Alternate pulsatile infusions of drug (pump 1) and buffer / saline (pump 2)
[0212] Total drug volume: 5mL
[0213] Total buffer volume: 8mL
[0214] Infusion rate of pump 1: 37 mL / min
[0215] Infusion rate of pump 2: 20 mL / min
[0216] Cycles: 5 cycles in the chest area
[0217]Time between loops: 10ms
[0218] Infusion Description: In the lumbar region, pump 1 infused 1 mL at 37 mL / min with a 10-ms pause, and pump 2 infused 1.6 mL at 30 mL / min with a 100-ms pause (cycle 1). This is repeated for a total of 5 cycles on the chest area.
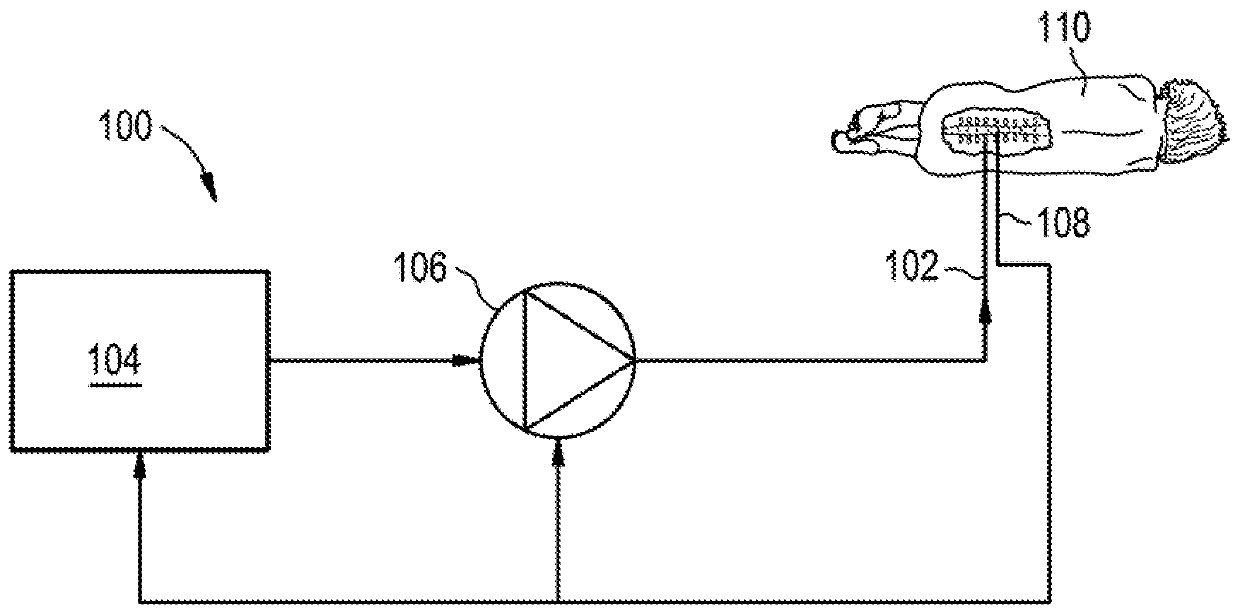
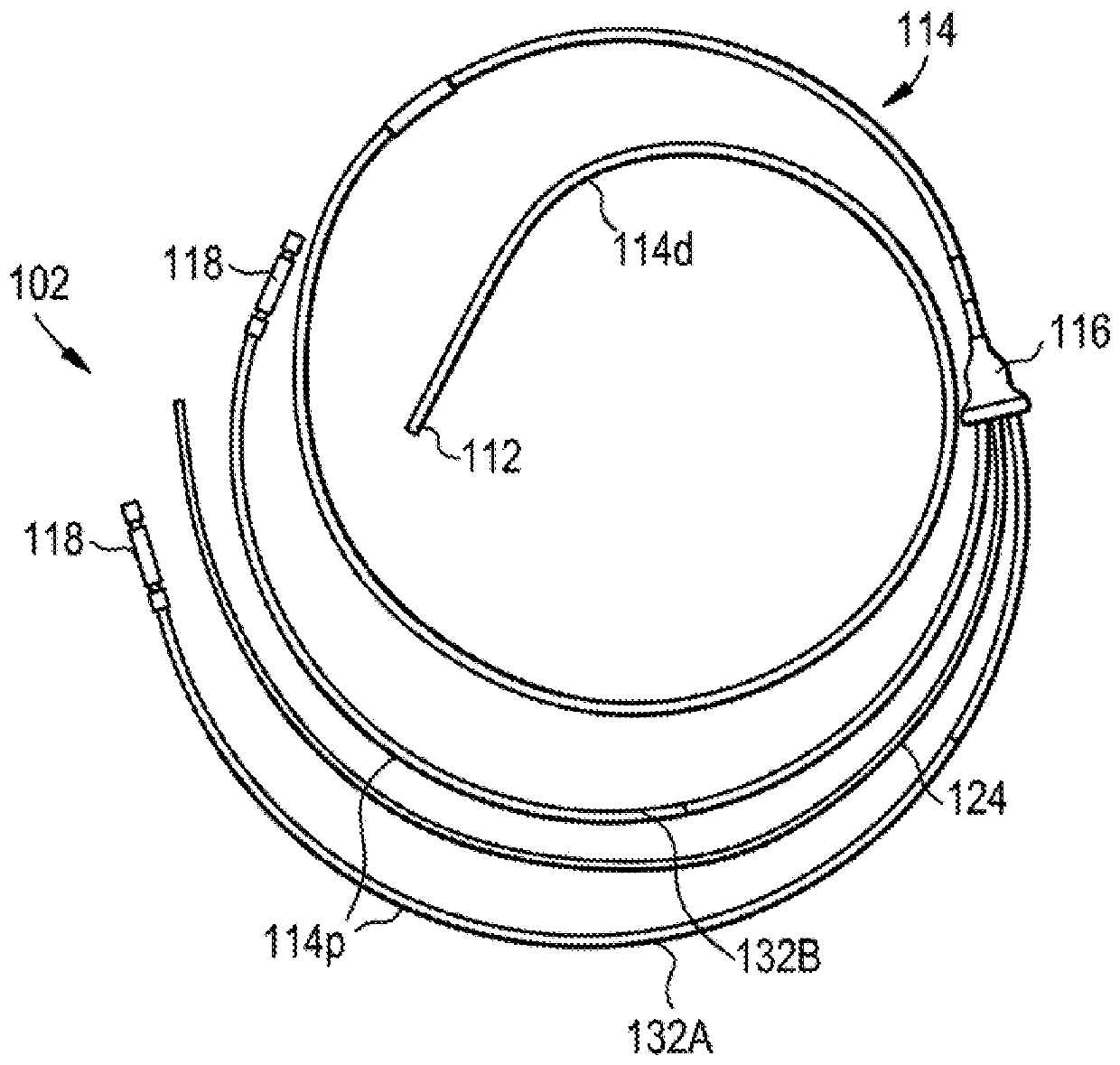
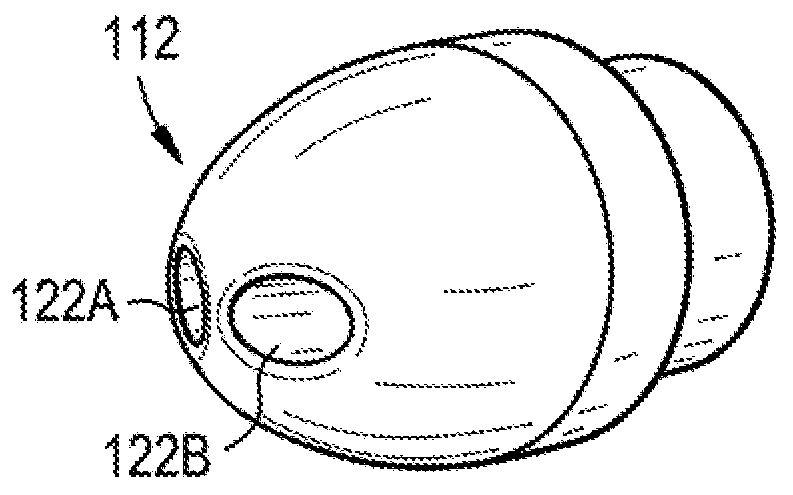
[0219] Figure 22 Drug delivery system 200 including lumbar puncture needle 292 is illustrated. Needle 292 may include a sensor 294 (eg, a pressure sensor) mounted adjacent the distal tip of the needle. Thus, as needle 292 is inserted into patient 210, sensor 294 may measure pressure or other characteristics of the patient's CSF. Needle 292 may also include an integrated or remote display 296 for displaying the output of sensor ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap