Methods of protecting vascular integrity induced by targeted radiation therapy
A radiotherapy and integrity technology that can be used in X-ray/γ-ray/particle irradiation therapy, pharmaceutical formulations, medical preparations with non-active ingredients, etc., and can solve problems such as unmet requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0083] The present invention also provides the following non-limiting embodiments.
[0084] Embodiment 1 is in a method of protecting vascular integrity in a subject exposed to targeted radiation therapy, the method comprising administering to the subject an effective amount of a thrombopoietin (TPO) mimetic.
[0085]Embodiment 1(a) is the method of embodiment 1, wherein the TPO mimetic comprises a peptide having the amino acid sequence of SEQ ID NO:1.
[0086] Embodiment 1(b) is the method of embodiment 1(a), wherein the peptide has the amino acid sequence of SEQ ID NO:2.
[0087] Embodiment 1(c) is the method of embodiment 1(a) or 1(b), wherein the TPO mimetic further comprises a hydrophilic polymer covalently linked to the peptide.
[0088] Embodiment 1(d) is the method of embodiment 1(c), wherein the hydrophilic polymer is any one of: i) polyethylene glycol (PEG), ii) polypropylene glycol, iii) polylactic acid, or iv) polyglycolic acid.
[0089] Embodiment 1(e) is the m...
Embodiment 1
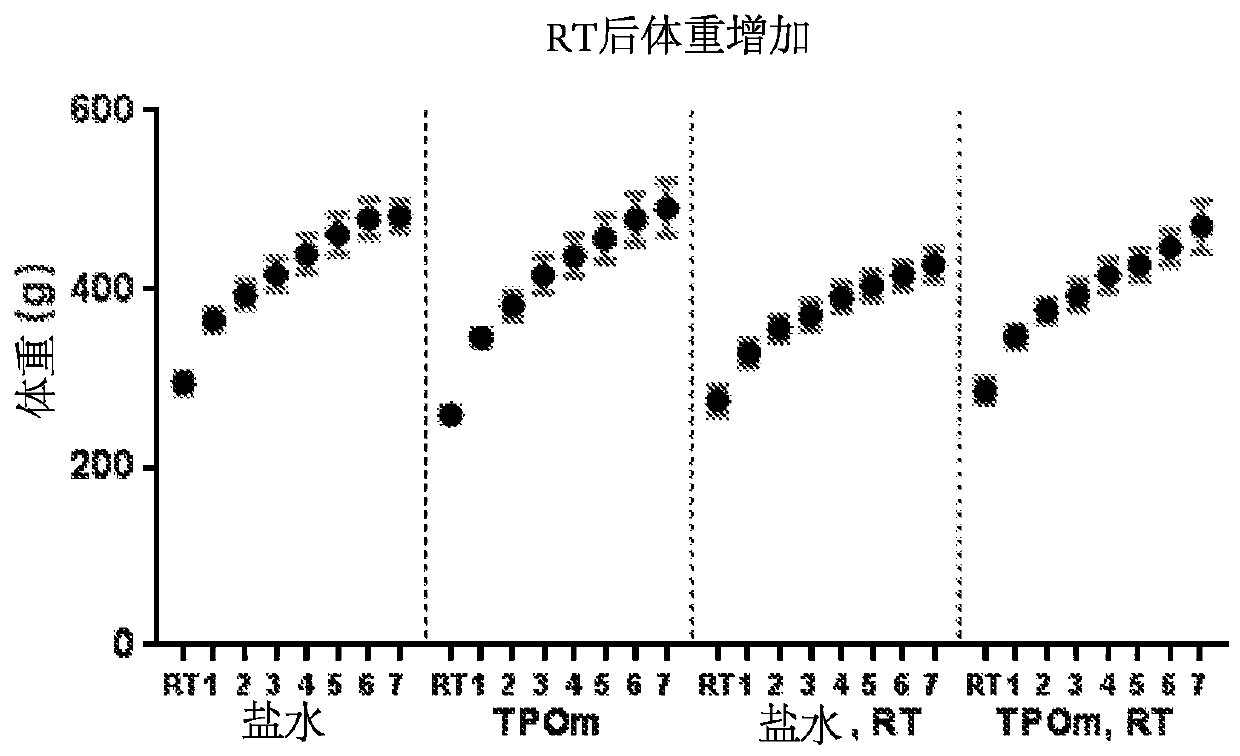
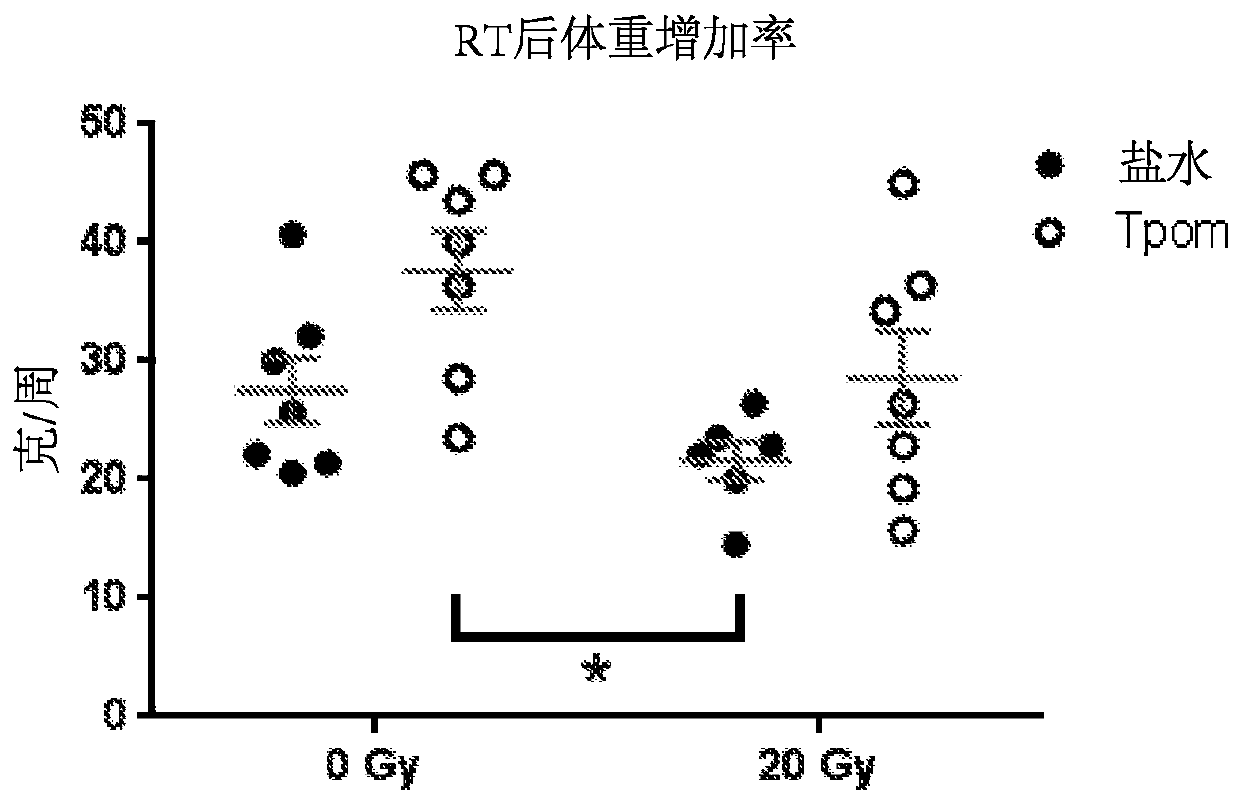
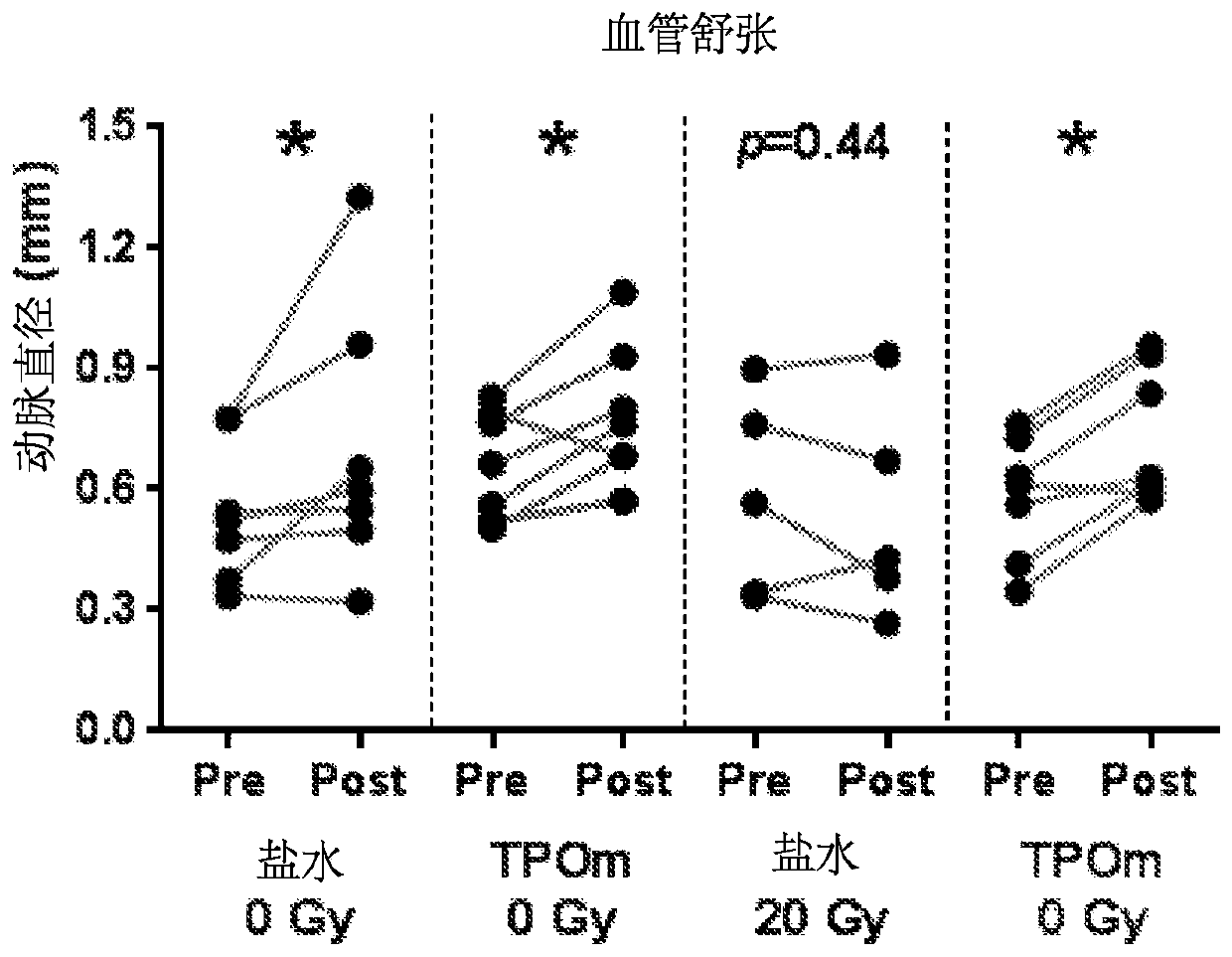
[0177] Example 1: Administration of Thrombopoietin Mimetics Preserves Vascular Function in Rat Prostate Irradiation Model of Erectile Dysfunction
[0178] Materials and methods
[0179] animal:
[0180] Thirty-five eight-week-old male Sprague Dawley rats were obtained from Charles River Laboratories (Malvern, PA) and were pair-housed under standard housing conditions. Animals were divided into 4 groups: saline / sham, saline RT, TPOm / sham, TPOm / RT. The Duke University School of Medicine Institutional Animal Care and Use Committee approved all animal work.
[0181] TPOm synthesis and treatment:
[0182] TPOm was synthesized by Janssen Pharmaceuticals as previously described (Knight et al., Int. J. Toxicol. 30(4):385-404 (2011)) and shipped as a lyophilized powder on dry ice to Duke University. TPOm was reconstituted in sterile saline, sterile filtered, aliquoted, and stored at -20°C until use. Ten minutes after RT, TPOm was administered as a single dose by subcutaneous inje...
Embodiment 2
[0202] Example 2: Evaluation of the effects of thrombopoietin mimetics on radiation-induced vascular permeability and leukocyte adhesion using a novel murine ear vein model
[0203] Materials and methods
[0204] animal
[0205] Flk1-mCherry mice were bred in the Duke University Breeding Core Facility (Larina et al., Anat. Rec. 292(3):333-41 (2009)). Throughout the experiment, male and female mice at least 8 weeks of age were transferred to an accessible facility and housed in groups. Vascular endothelial cells of Flk1-mCherry mice constitutively express mCherry, which facilitates intravital microscopy imaging and analysis. All animal work was approved by the Institutional Animal Care and Use Committee at Duke University School of Medicine.
[0206] ear irradiation
[0207]Mice were anesthetized by intraperitoneal injection of 80 / 8 mg / kg ketamine / xylazine. Mice were placed in a cylindrical lead jig (jig) that shielded the entire body except the pinna of the left ear, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com