Chimeric antigen receptor and application thereof
A technology of chimeric antigen receptors and antigens, applied in medicine, in the field of chimeric antigen receptors, can solve problems such as low expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0083] Example 1 Design and function of BAFF antigen-binding domain
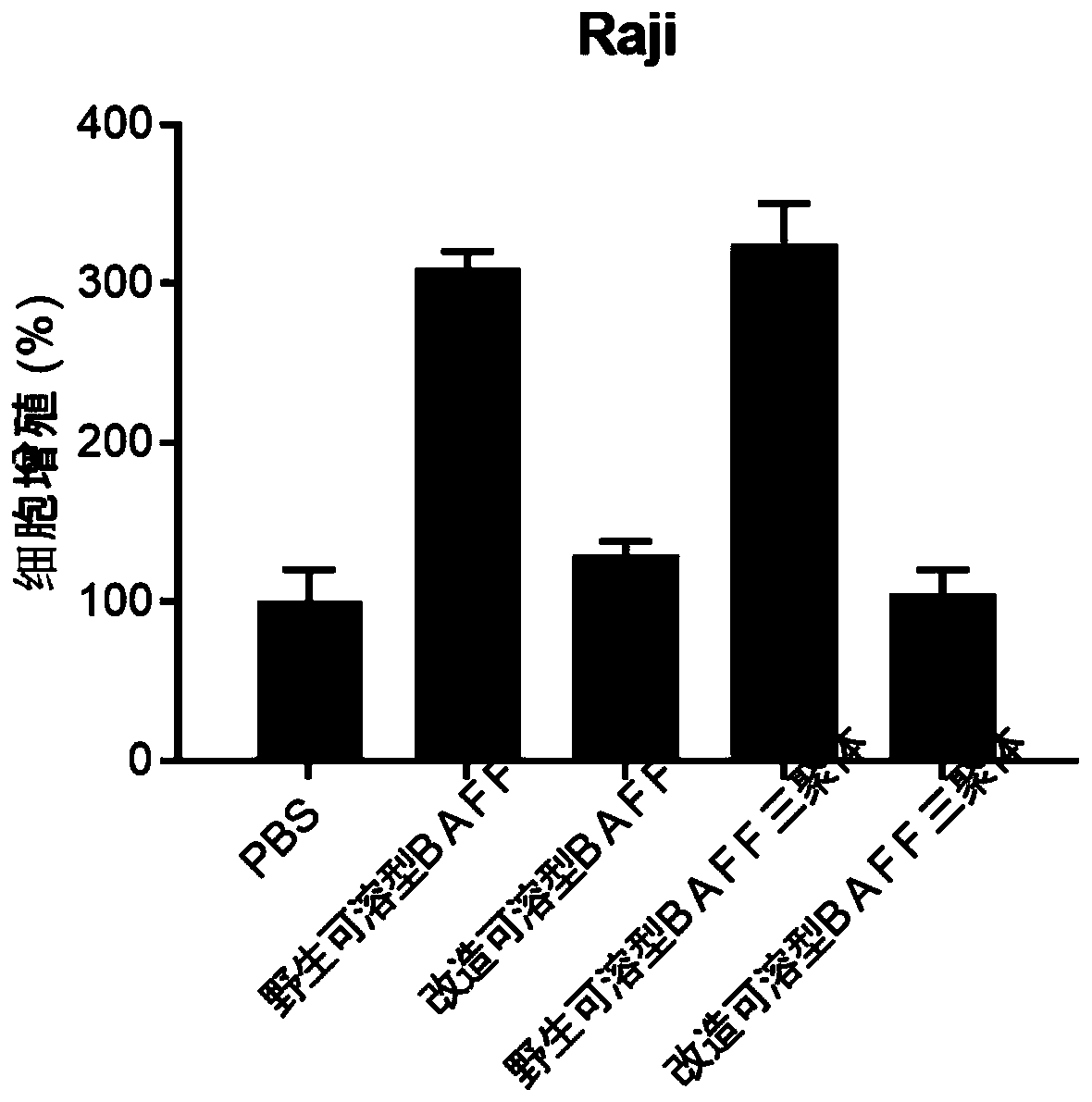
[0084] In order to use BAFF as the antigen-binding domain of the CAR molecule, the soluble BAFF was modified in this example, and the amino acid VHVFGDEL, which is necessary for the activation of positions 217-224 of the soluble BAFF (soluble form: 134-285), was replaced with GG, The modified BAFF shown in SEQ ID NO:1 was obtained.
[0085] (1) Binding ability of modified BAFF to BAFF-R
[0086] The results are shown in Table 1. The modified BAFF (mBAFF) lost the BAFF activation ability, but retained the BAFF-R binding ability.
[0087] Table 1 Analysis of the binding of recombinant BAFF and modified protein to K562-BAFFR cells
[0088] target protein Mean Fluorescence Intensity (MFI) binding positive rate wild soluble BAFF 856 59.35% Transformation of soluble BAFF 969 62.56% Wild soluble BAFF trimer 2968 97.45% Modified soluble BAFF trimer 2565 95.67% PBS 25...
Embodiment 2
[0092] Embodiment 2 Design of CAR molecules
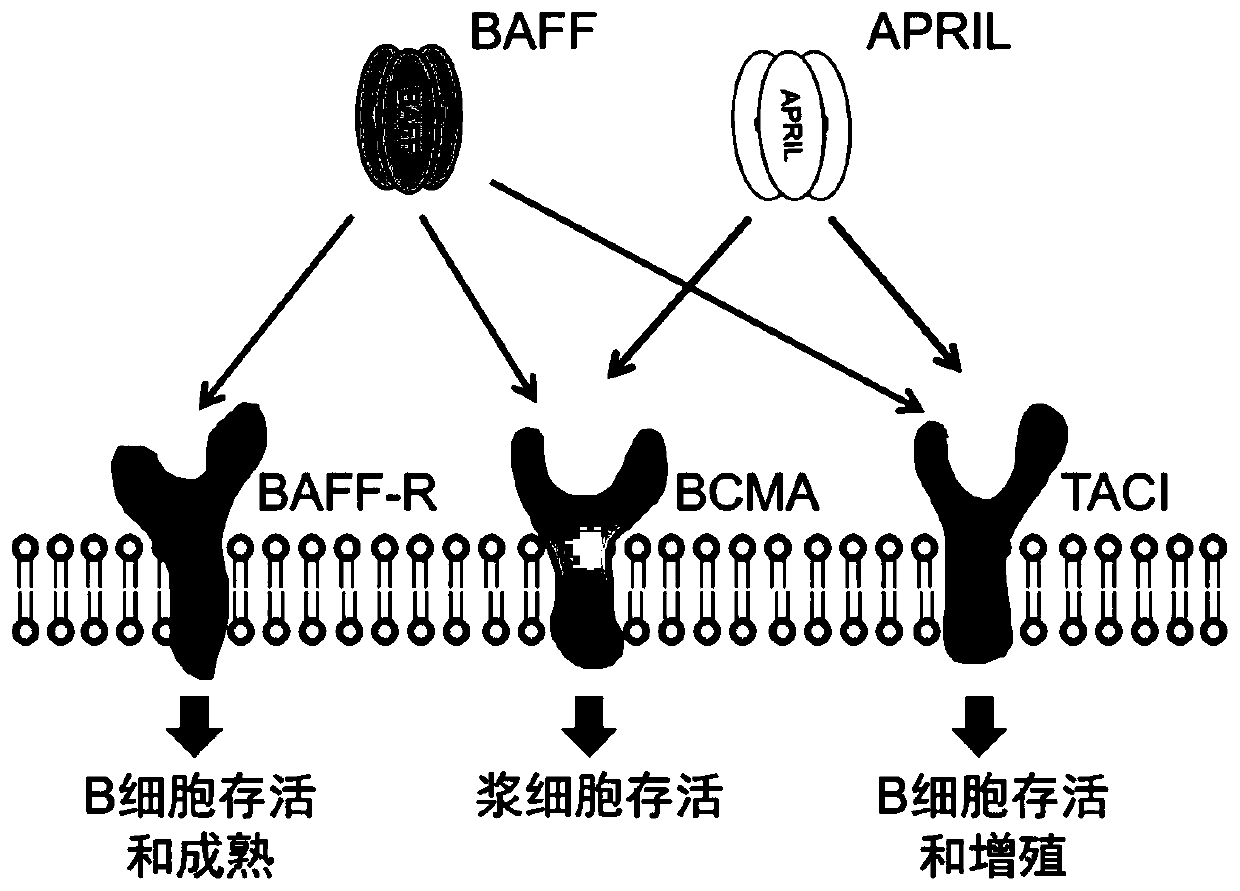
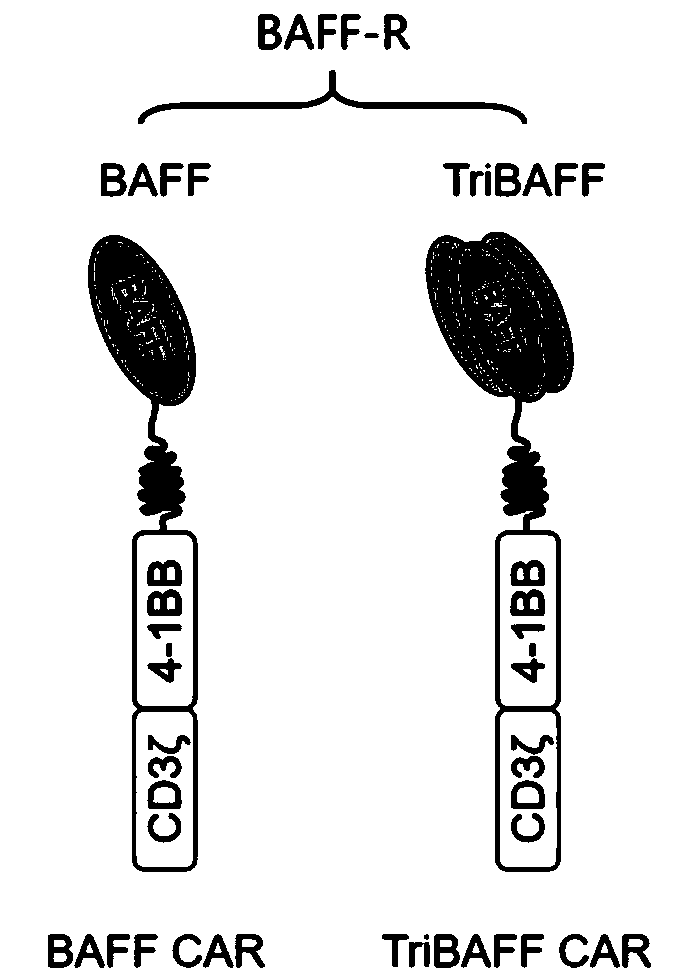
[0093] In this example, the engineered BAFF shown in SEQ ID NO: 1 is used as the antigen-binding domain to construct a monomeric BAFF-based chimeric antigen receptor (BAFF CAR) as shown in Figure 3 (A) and a trimeric BAFF-based The chimeric antigen receptor (TriBAFF CAR) of the body BAFF (TriBAFF CAR), the three BAFFs in the TriBAFF CAR molecule are connected by a GGGGS linker, which recognizes and binds to BAFF-R; The CD19 CAR of the CD19 antibody (FMC63) scFv specifically recognizes the CD19 antigen.
[0094] In addition to the antigen-binding domain, the above-mentioned CAR molecule also includes a CD8α signal peptide sequence (Leader), a CD8α hinge region (Hinge) and a transmembrane region (Transmembrane) sequence, a 4-1BB co-stimulatory domain sequence and a CD3ζ signaling domain sequence.
Embodiment 3
[0095] The construction of embodiment 3 lentiviral vectors
[0096] (1) Whole gene synthesis of nucleic acid molecules of the following CAR molecules:
[0097] BAFF CAR: composed of CD8a signal peptide sequence, modified BAFF sequence, CD8a hinge region and transmembrane region sequence, 4-1BB and CD3ζ in tandem (the amino acid sequence is shown in SEQ ID NO: 8);
[0098] TriBAFF CAR: consists of CD8a signal peptide sequence, three repeats of modified BAFF sequences (connected by GGGGS linker between the three BAFFs), CD8a hinge region and transmembrane region sequence, 4-1BB and CD3ζ in series (amino acid sequence such as Shown in SEQID NO:9);
[0099] CD19 CAR: composed of CD8a signal peptide sequence, CD19 scFv, CD8a hinge region and transmembrane region sequence, 4-1BB and CD3ζ in tandem (the amino acid sequence is shown in SEQ ID NO: 10);
[0100] SEQ ID NO: 10:
[0101] Amino acid sequence of CD19 CAR:
[0102] MALPVTALLLPLALLLHAARPDIQMTQTTSSLSASLGDRVTISCRASQDISKYLNW...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com