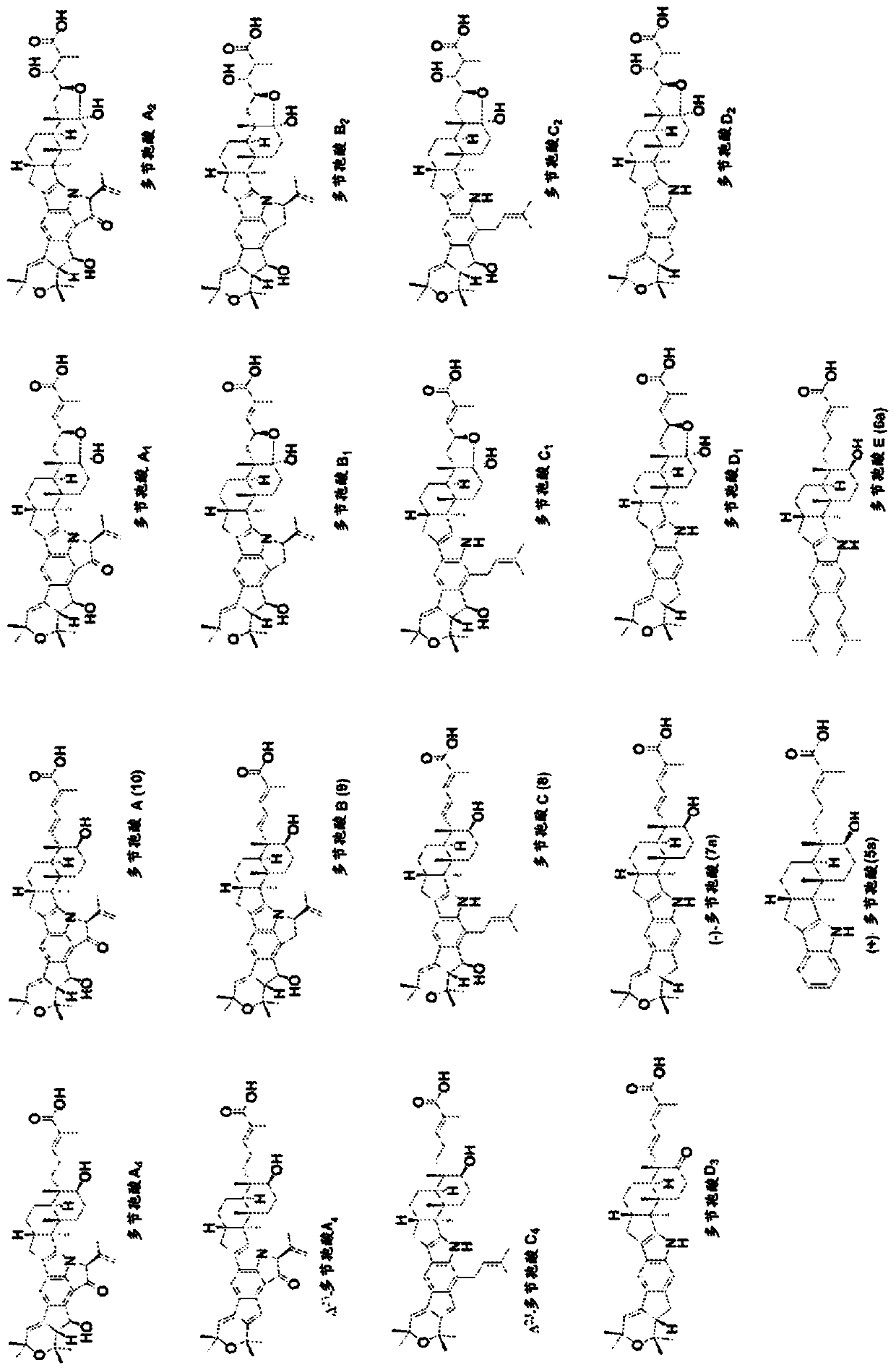
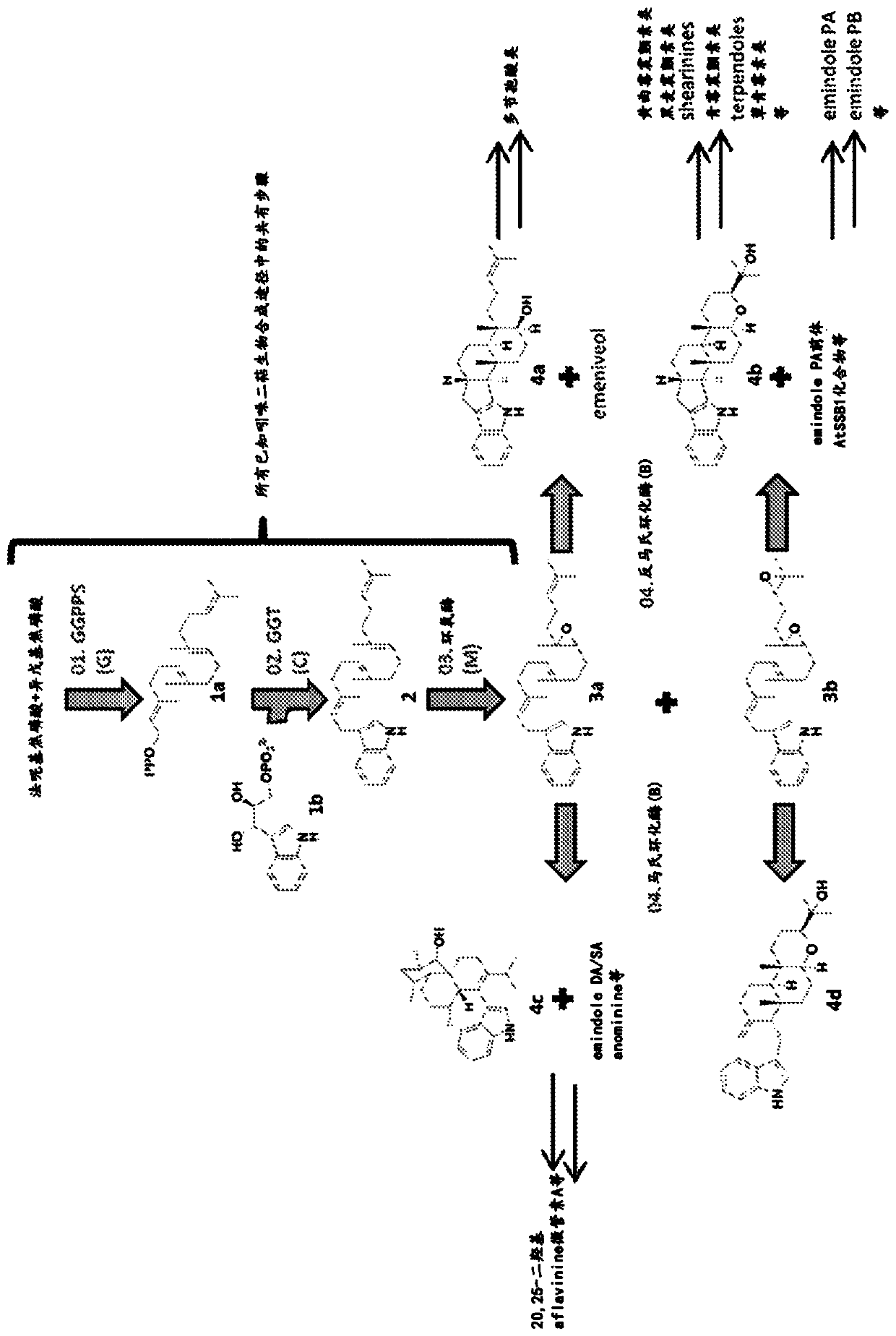
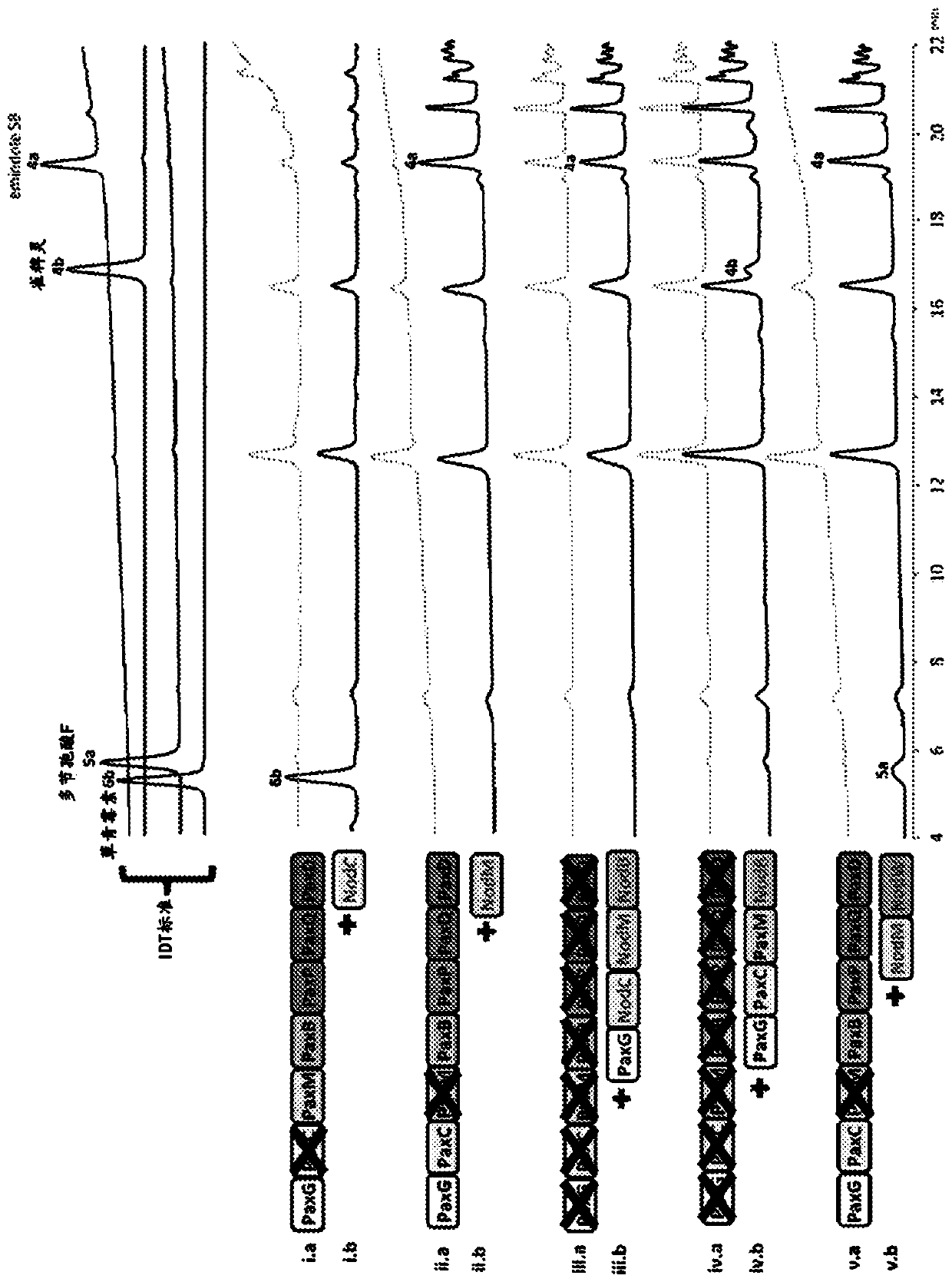
Heterologous biosynthesis of nodulisporic acid
A biosynthesis and polynucleotide technology, applied in the direction of microorganisms, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems that NAA10 production cannot be realized, NAA10 biosynthesis is difficult, and total synthesis has not yet been realized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0274] Materials and methods
[0275] gDNA Isolation for Genome Sequencing and TUM Amplification
[0276] Genomic DNA for genome sequencing and PCR amplification of TUM, following the method of Byrd et al. 26 And make a modification from fungus Penicillium paxilli (Penicillium paxilli) 26601 TM (PN2013) 24 and Hypoxylonpulicicidum strains 74245 TM,25 separate. Prepare Milli- Sterile 2.4% (w / v) Difco in water TM Potato dextrose broth (Becton, Dickinson andCompany, Maryland, U.S.A.), and 5 × 10 6 spore or ~1cm 2 Freshly ground mycelia (strains used for asporulation) were inoculated. The culture was incubated at 22°C with shaking (200 rpm) for 2-4 days. The fermentation broth was filtered through sterile diaper liners, and the mycelium was washed three times with sterile water. Transfer the mycelium to a sterile 15 mL centrifuge tube and snap freeze in liquid nitrogen to lyophilize for 24-48 h. 15-20 mg of freeze-dried mycelia were placed in a mortar containing l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com