N-type neutral double-free-radical conductive compound as well as preparation method and application thereof
A compound and selected technology, applied in the direction of organic chemistry, can solve the problem that quinone diradicals have not been considered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of 2DQQT-Se
[0074]
[0075] 1) Preparation of compound (III-1): Dissolve compound (IV-1) (100mg, 0.11mmol) and (V-1) (148mg, 0.264mmol) in dry toluene (2mL) and DMF (2mL) In the mixed solvent, add it to the pressure-resistant tube under inert conditions, and ventilate for 20 minutes to remove oxygen. Add tetrakis triphenylphosphine palladium [Pd (PPh 3 ) 4 ] (6.36 mg, 0.0055 mmol). Wherein compound (IV-1) and compound (V-1) and Pd(PPh 3 ) 4 The molar ratio is 1:2.4:0.05. Stir at 90°C for two days, cool to room temperature after the reaction, remove the solvent, and purify the crude product by silica gel column chromatography to obtain 134 mg of red solid compound (III-1), with a yield of 90% .
[0076] 2) Preparation of compound (II-1): Add compound (III-1) (134 mg, 0.099 mmol) into a single-necked bottle under light-shielded conditions. Chloroform (3 mL) and DMF (1 mL) were added to dissolve, and then NBS (39 mg, 0.219 mmol) was ad...
Embodiment 2
[0078] Embodiment 2: Preparation of 2DQQT-S
[0079]
[0080] Referring to the preparation method of Example 1, 2DQQT-S can be prepared with a yield of 50%. HRMS (MALDI-TOF) molecular formula: C 74 h 94 N 6 o 4 S 4 S 2 . [M+H] + Theoretical value: 1322.565533, measured value: 1322.564814.
Embodiment 3
[0081] Embodiment 3: performance test
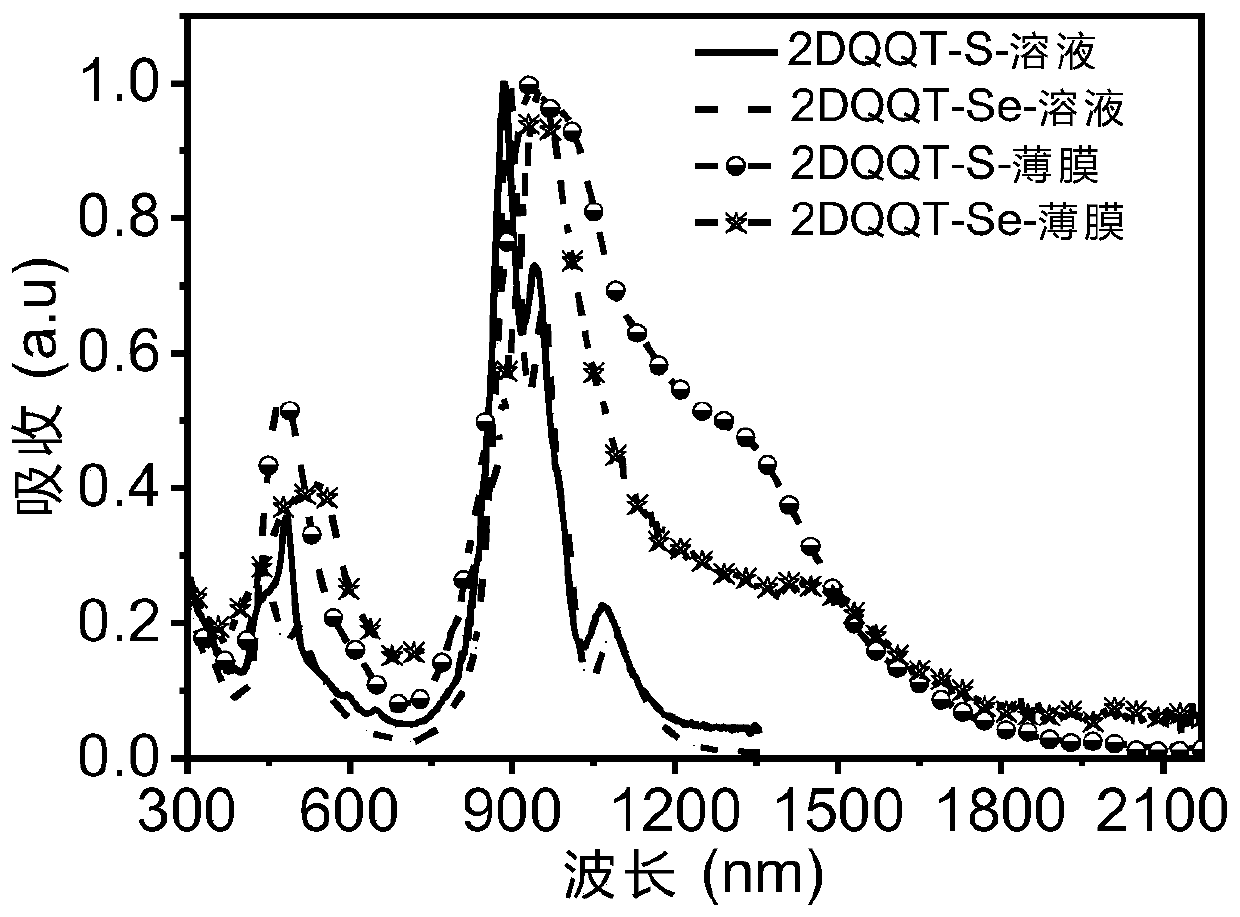
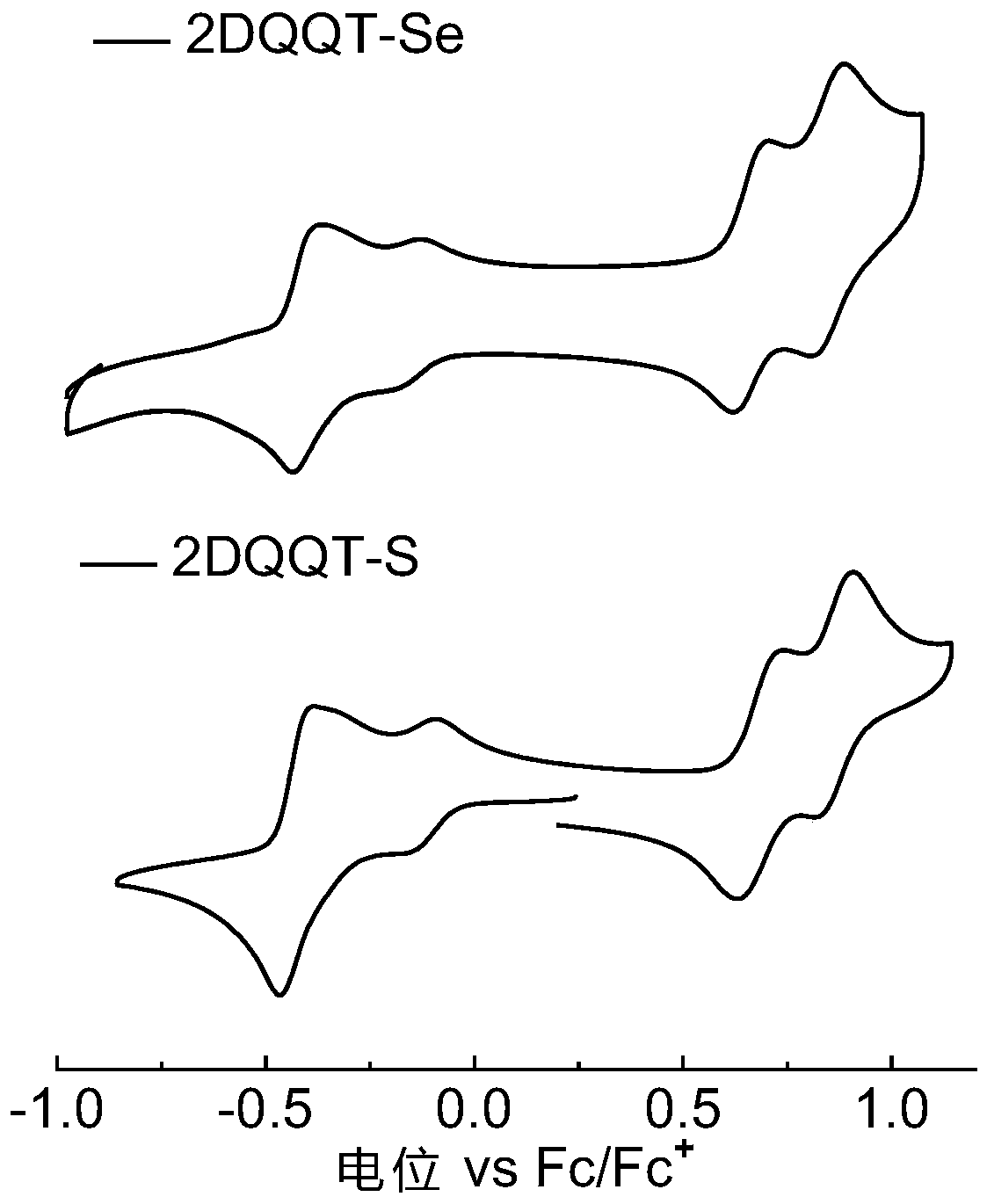
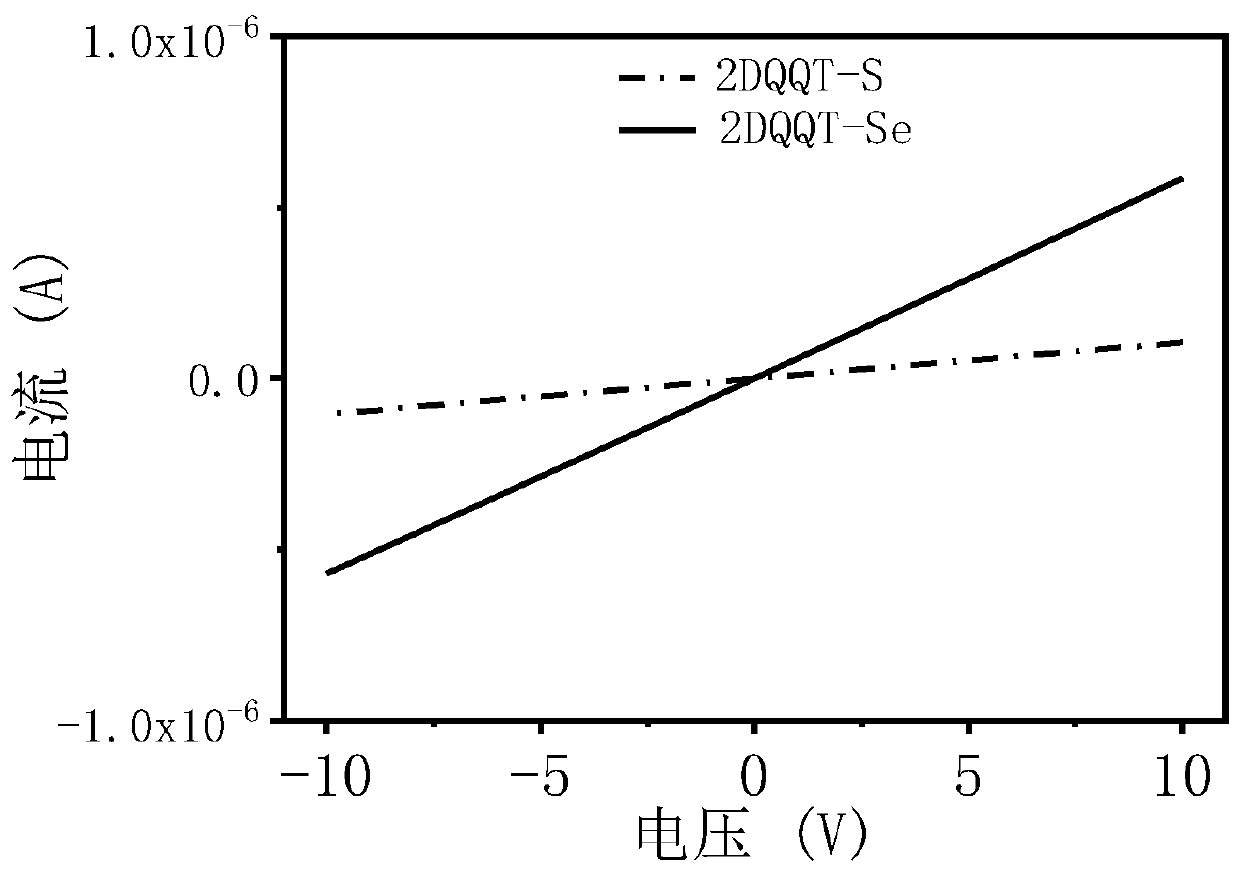
[0082] The compound 2DQQT-Se and the compound 2DQQT-S in the above examples were respectively dissolved in dichloromethane (about 0.00001mol / L) and spin-coated into a thin film, and tested at room temperature using a UV-visible spectrophotometer. See results figure 1 . It can be seen that the solutions of the two compounds both exhibit strong absorption in the near-infrared region. The absorption of the film has a significant red shift compared with that of the solution, and a shoulder peak is generated in the wavelength range of 1300-1500nm, resulting in a very narrow band gap. The surface molecules can generate quinone and aromatic resonances at room temperature, and self-doping, This point can be further proved by combining theoretical calculations, energy spectra and single crystal data. In addition, in the 2DQQT-Se film, there is a broad charge vibration peak absorption, which corresponds to its high doping degree, which also pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com