A method for obtaining a highly efficient binding substrate sequence of adar protein in cells, as well as the substrate sequence and application
A protein and substrate technology, applied in fixed-point targeted RNA editing, locating the substrate sequence information that ADAR protein binds efficiently in cells, and obtaining field, can solve the problem of inability to effectively use ADAR protein to act on substrate information acquisition, and it is difficult to obtain widely The clinical application and the low proportion of chimeras can achieve the effects of shortening the experimental time, increasing the yield, and increasing the number of constructions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Construction of ADAR1 Protein High Affinity Substrate Sequence and RNA Level Targeted Editing in HEK293 Cell Line
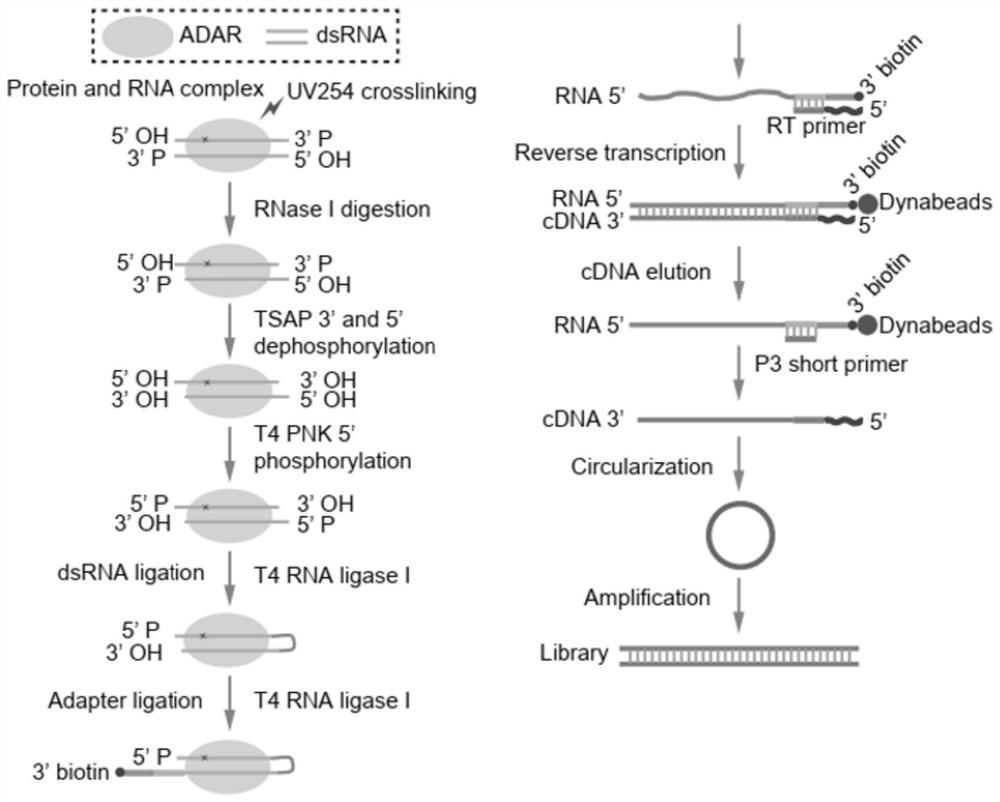
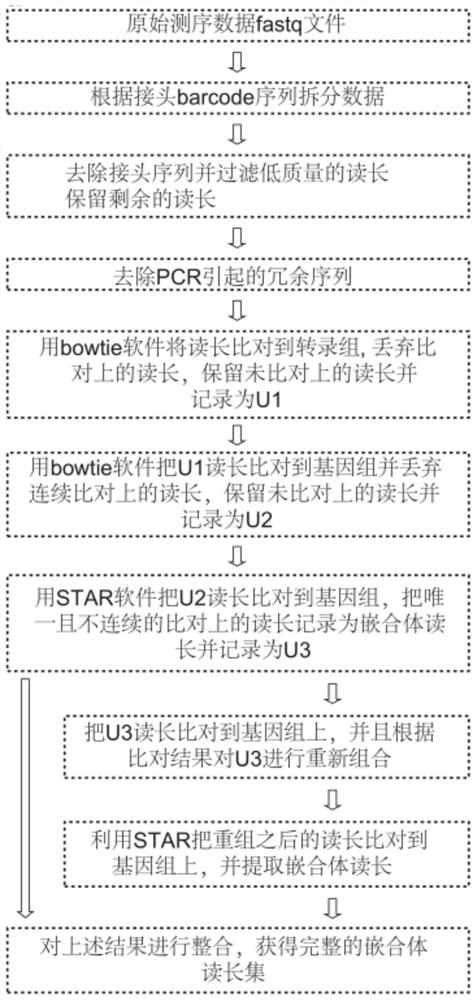
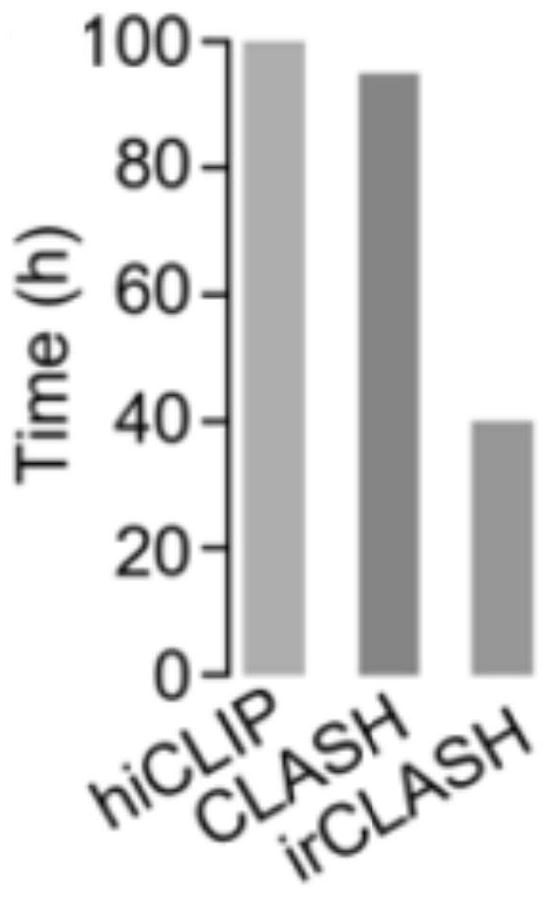
[0042] The overall process of the experimental method of the present invention is as follows: figure 1 As shown, the experimental sequencing library data analysis process is as follows figure 2 As shown, compared with other published methods, the results show that the method of the present invention greatly shortens the time-consuming experiments (such as image 3 ); the present embodiment takes the ADAR protein family ADAR1 protein as an example to further illustrate the present invention; specifically, the method includes the following steps:
[0043] 1. Cell preparation and protein and RNA cross-linking reaction
[0044] HEK293 cells were cultured in a 15 cm cell culture dish and overexpressed FLAG-tagged ADAR1. After 48 hours, the cells were washed with PBS and the cross-linking reaction of protein and RNA was performed on a 254 nm ultravi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com