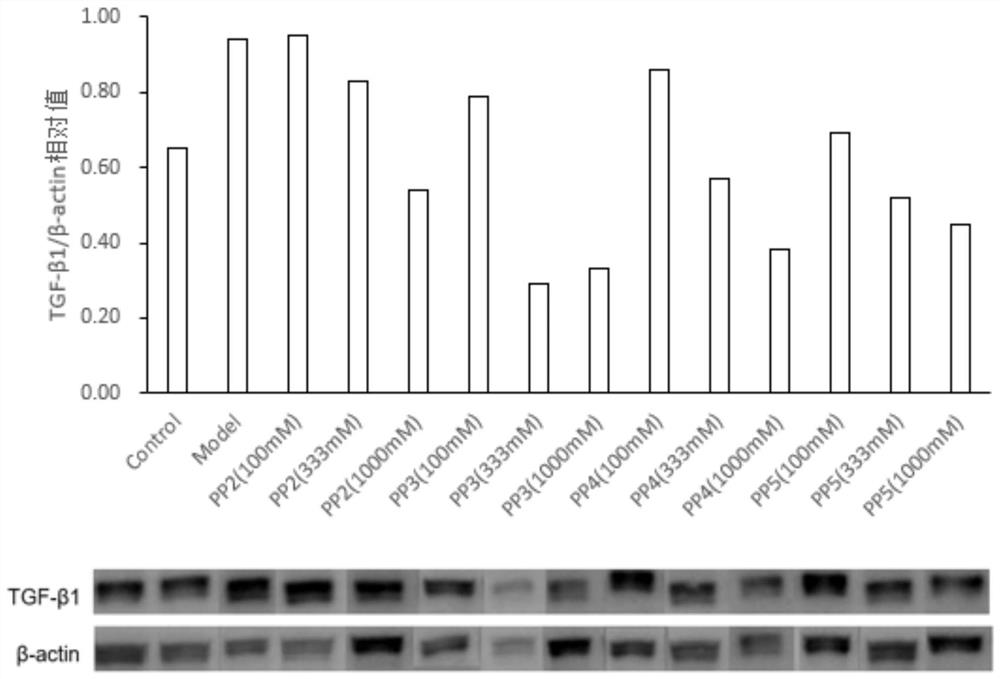
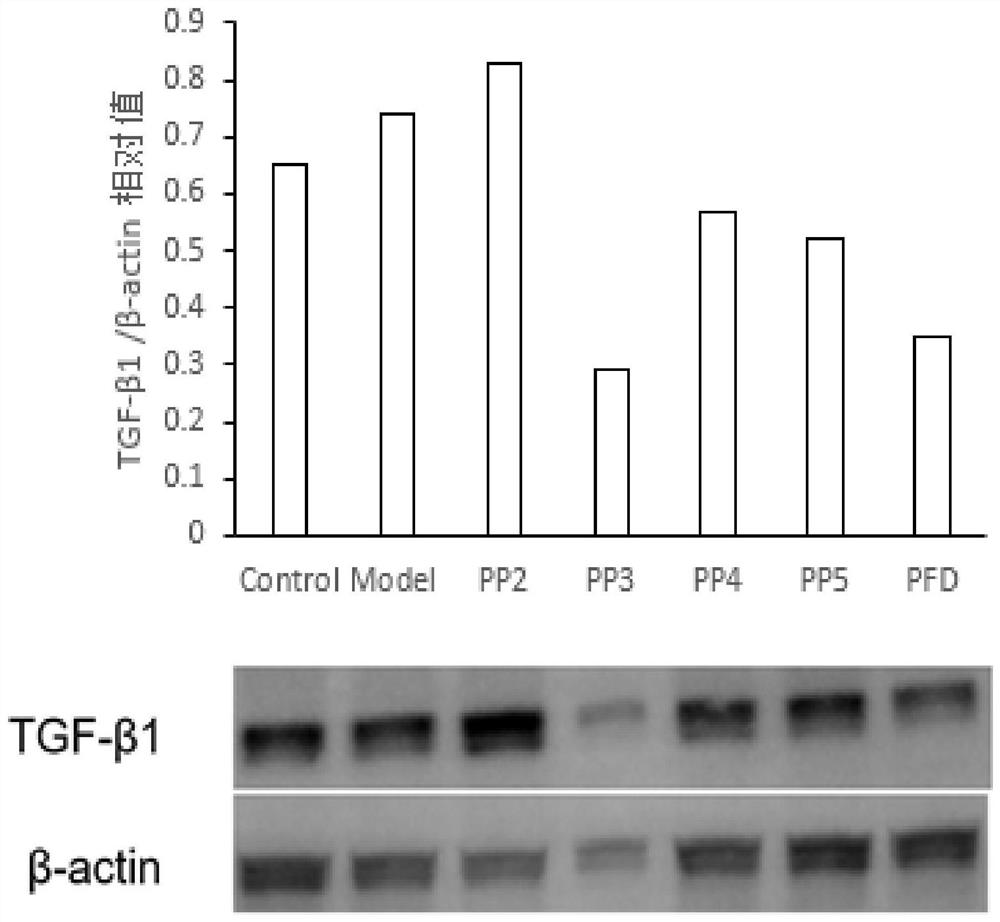
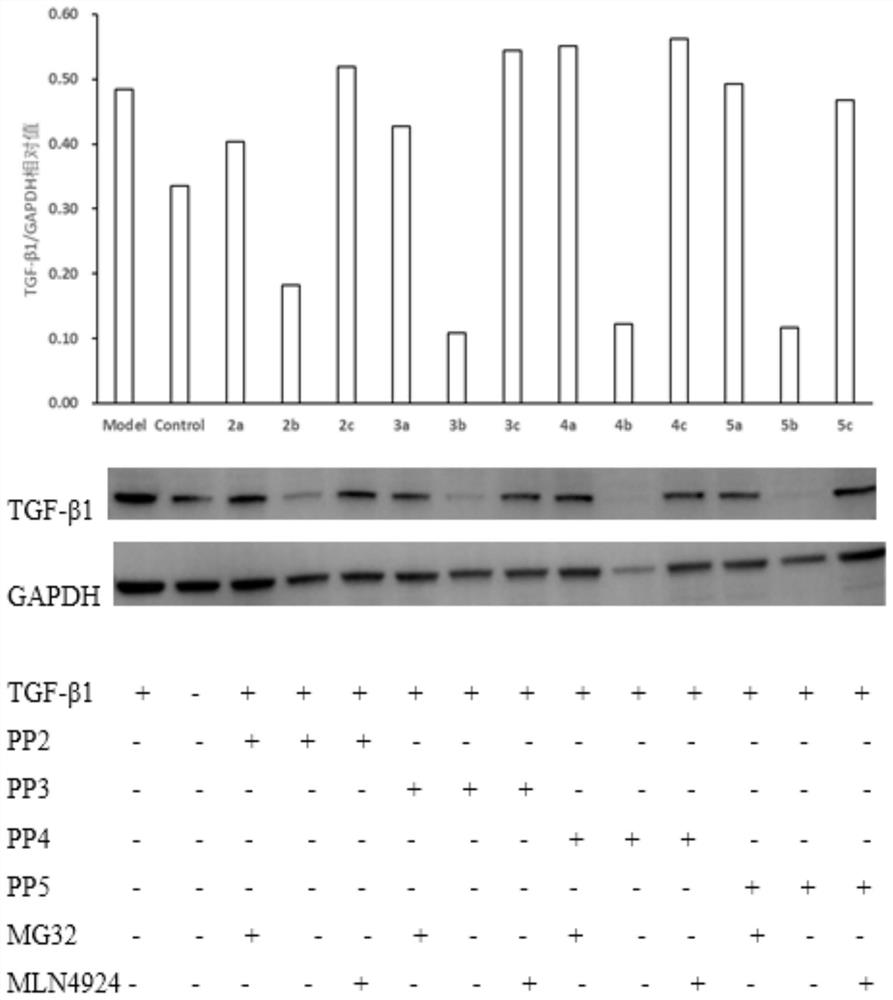
A compound targeting ubiquitination to degrade TGF-β1 and its preparation method and application
A technology of TGF- and compounds, applied in the fields of organic chemistry, drug combination, respiratory diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of TGF-β small molecule ligand
[0043] 1-(4-hydroxyl-phenyl)-5-methyl-1H-pyridin-2-one (II, R is CH 3 )
[0044] According to the method disclosed in the Chinese invention patent with the application number 200810201706.9, it is prepared. m.p.167-169°C. 1 HNMR (δ, CDCl 3 ): 7.34-7.37 (m, 1H), 7.14 (s, 1H), 6.97-7.00 (d, 2H), 6.68 (s, 1H), 6.62-6.66 (m, 2H), 2.12 (s, 3H).
[0045]
Embodiment 2
[0046] Example 2 Preparation of E3 Ligase Ligand
[0047] (1) Hydroxythalidomide (IV a)
[0048]
[0049] 10.0 g (0.06 mol) of 3-hydroxyphthalic anhydride, 10.0 g (0.06 mol) of 3-amino-2,6-piperidinedione hydrochloride and 100 mL of toluene were successively added into the three-necked flask. Under the protection of nitrogen, the reaction was heated under reflux for 12 hours, a gray solid was precipitated, and the reaction was monitored by TLC. Cool to room temperature, suction filter and dry to obtain 12.0 g off-white powder. Yield 73%. HRMS m / z(ESI)calcd for C 13 h 10 N 2 o 5 [M+Na] + :297.0487; found: 297.0489. 1 H NMR (400MHz, DMSO-d 6 )δ11.21(s,1H),11.13(s,1H),7.66(dt,J=11.7,5.9Hz,1H),7.37-7.30(m,1H),7.27(d,J=8.4Hz,1H ),5.10(dd,J=12.8,5.4Hz,1H),3.05-2.78(m,1H),2.59(ddd,J=21.9,14.5,11.0Hz,2H),2.16-1.87(m,1H). 13 C NMR (100MHz, DMSO-d 6 ) δ 173.30, 170.51, 167.65, 166.30, 155.93, 136.84, 133.61, 124.47, 124.02, 114.80, 49.11, 31.44, 22.51.
[0050] (2) Propa...
Embodiment 3
[0053] The preparation of embodiment 3 connecting chain
[0054] (1) Azidodiethylene glycol p-toluenesulfonate (1)
[0055]
[0056] Add 415 mg (1 mmol) of diethylene glycol di-p-toluenesulfonate and 65 mg (1 mmol) of sodium azide into the three-necked flask respectively. Add 10 mL of anhydrous DMF, and stir at 60° C. for 4 h under nitrogen protection. TLC monitored the end of the reaction. Cool to room temperature, add 40 mL of cold water, extract several times with a small amount of ethyl acetate, dry over anhydrous sodium sulfate, filter, combine and concentrate the filtrate to obtain a yellow oil. 80 mg of light yellow liquid was obtained by column chromatography, yield: 28%. HRMS m / z(ESI)calcd for C 11 h 15 N 3 o 4 S[M+Na] + :308.0681; found: 308.0688.
[0057] (2) Azide triethylene glycol p-toluenesulfonate (2)
[0058]
[0059] Refer to Example 3(1) for the operation process, and use triethylene glycol di-p-toluenesulfonate instead of diethylene glycol d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com