Conjugates of biomolecule and use thereof
A technology of biomolecules and conjugates, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as patient death and severe pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1: Synthesis of chemical structures for activatable and binding arms
[0178] When R2 has the amino acid sequence of Ala-Ala-Asn and R3 is PABC (R3-5), the synthetic route is as follows:
[0179]
[0180] When R1 and R4 are different substituents, the following compounds shown in Table 1 are obtained.
[0181] Table 1
[0182]
[0183]
[0184]
[0185]
[0186] As shown in S15, the specific synthesis process is as follows:
[0187]
[0188] 1) Fmoc-Asn(Trt)-OH (20g, 0.03mol),
[0189] Add 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) (15g, 0.04mol) and DMF (200mL) into a three-necked flask and stir for 30 minutes. p-Aminobenzyl alcohol (4.1 g, 0.03 mol) and N,N-diisopropylethylamine (8.7 g, 0.06 mol) were added at 0°C, respectively, followed by stirring at room temperature for 3 hours. Most of the DMF was removed by rotary evaporation. The residue was dissolved in ethyl acetate (200 mL), washed successive...
Embodiment 2
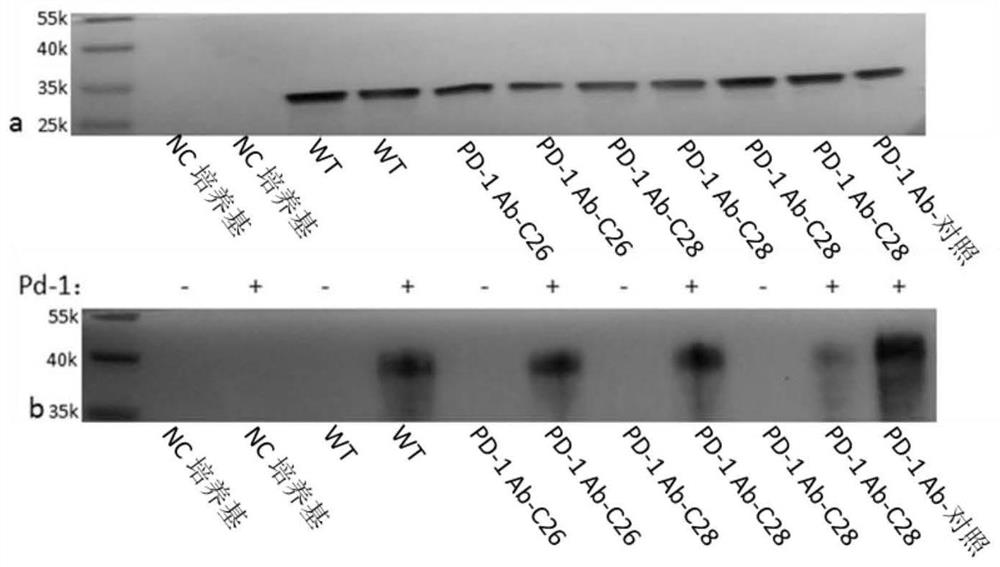
[0230] Example 2: Analysis of the binding activity of the CDR mutation of the antibody variable region and screening for R4
[0231]The amino acid sequence of the anti-PD-1 antibody disclosed in WO200815712A1 and its DNA sequence were optimally expressed and synthesized in the host (Jinweizhi). The amino acid sequence and DNA sequence of the anti-PD-1 antibody 2 disclosed in WO2006121168A2 were optimally expressed and synthesized in the host (Jinweizhi). The amino acid sequence and DNA sequence of the anti-CTLA-4 antibody disclosed in US20150283234 were optimized for expression and synthesis in the host (Jinweizhi). The amino acid sequence of the anti-TNFα antibody disclosed in US009534046 and its DNA sequence were optimized for expression and synthesis in the host (Jinweizhi). The amino acid sequence of the anti-CD-28 antibody disclosed in US007939638 and its DNA sequence were optimally expressed and synthesized in the host (Jinweizhi). The synthetic DNA was digested and li...
Embodiment 3
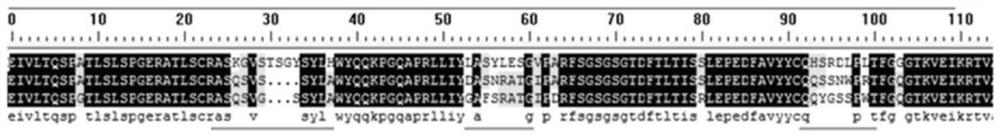
[0277] Example 3: Analysis and Screening of Binding Activity of Mutants with High Homology Sequence Mutations in Non-CDRs of Variable Regions R1
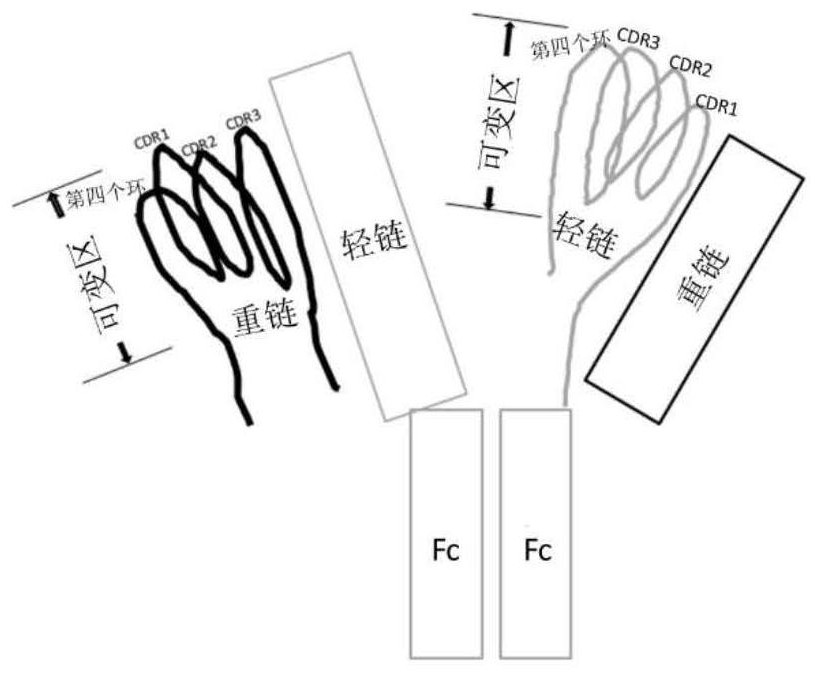
[0278] An antibody consists of 4 peptide chains, including two identical light chains and identical heavy chains. Two chains form a single monomer through disulfide bonds and non-covalent bonds. There are two types of light chains, such as κ and λ, and 5 types of light chains, such as μ, δ, γ, ε, and α. An antibody, as a whole, is divided into constant and variable regions. The variable regions are located at the ends of the two arms of the Y-shaped structure. Humanized or humanized antibodies have a certain degree of universality, and they all contain 4 loops (attached image 3 ). The three loops are highly variable and can directly bind to the antigen. These regions in the loop are called CDR regions, where CDR1, CDR2 and CDR3 are respectively in the three loops. On the same side there is another loop where the antibody binds ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com