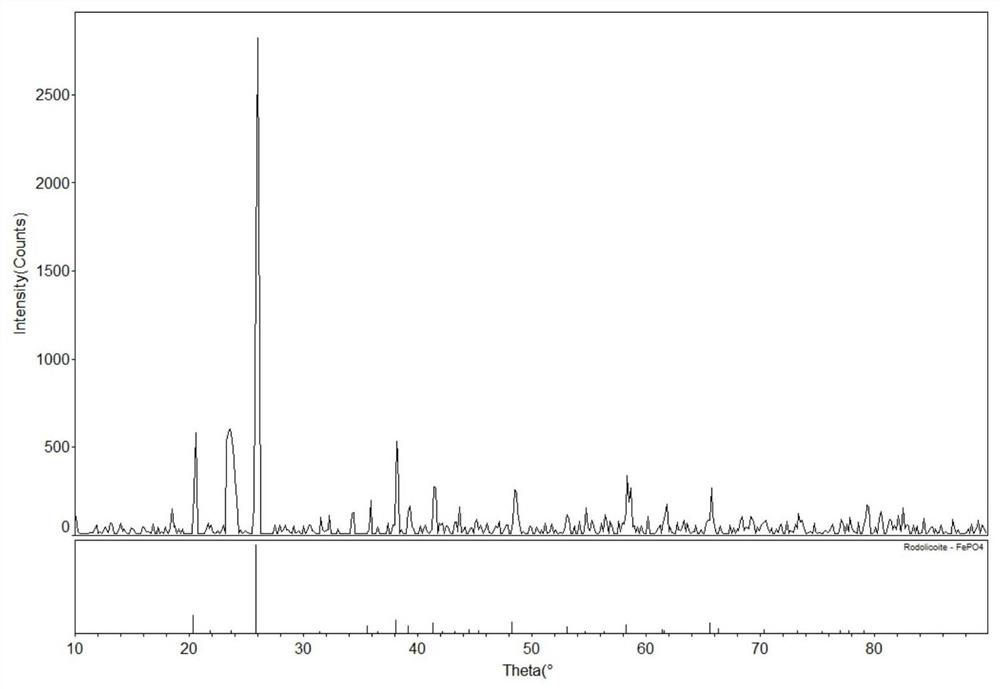
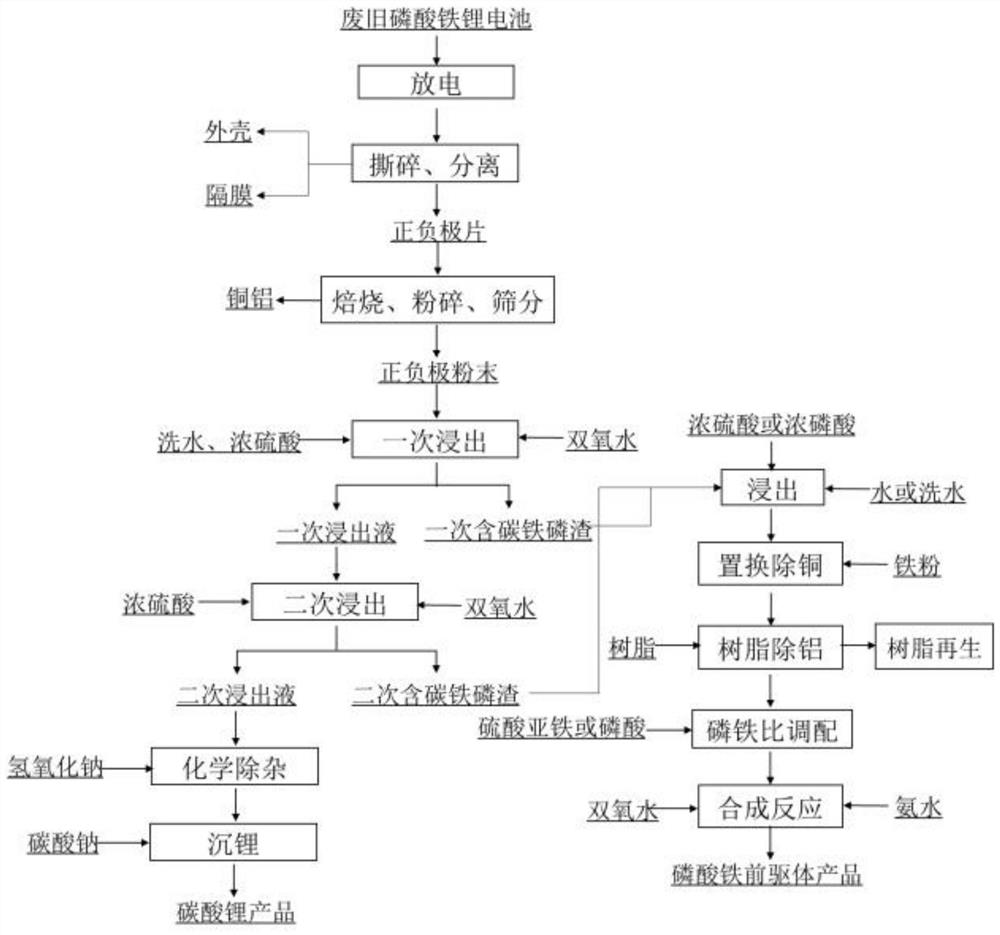
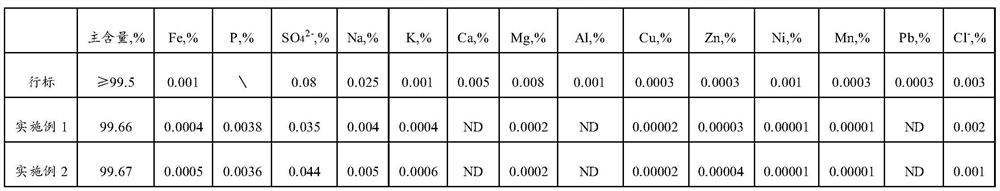
Method for recovering lithium from waste lithium iron phosphate batteries and method for recovering lithium and iron phosphate
A lithium iron phosphate battery and lithium recovery technology, applied in the field of lithium ion batteries, can solve the problems of long overall process, complex process and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] 1. Recycling and producing lithium carbonate from waste lithium iron phosphate batteries
[0161] a) Discharge, shred, and separate the waste lithium iron phosphate battery to obtain the shell, separator, and positive and negative electrodes; roast the positive and negative electrodes in a nitrogen atmosphere at 350°C, crush and sieve to obtain copper aluminum and positive and negative electrode powder . Among them, copper and aluminum are separated into copper powder and aluminum powder through variable-diameter dry separation columns for recycling. The positive and negative electrode powders and washing water were mixed at a solid-to-liquid ratio of 1:1 for slurrying, and reacted for 10 minutes to obtain slurrying liquid 1.
[0162] b) Add concentrated sulfuric acid (mass fraction: 98%) and hydrogen peroxide (concentration: 30%) to slurry mixing liquid 1, wherein, the amount of hydrogen peroxide added is 3 times of the theoretical amount, and concentrated sulfuric ac...
Embodiment 2
[0178] 1. Recycling and producing lithium carbonate from waste lithium iron phosphate batteries
[0179] a) Discharge, shred and separate the waste lithium iron phosphate battery to obtain the shell, diaphragm and positive and negative electrodes; roast the positive and negative electrodes at 600°C in a nitrogen atmosphere, crush and sieve to obtain copper aluminum and positive and negative electrode powder . Among them, copper and aluminum are separated into copper powder and aluminum powder through variable-diameter dry separation columns for recycling. The positive and negative electrode powders and washing water were mixed at a solid-to-liquid ratio of 1:2 for slurrying, and reacted for 10 minutes to obtain slurrying liquid 1.
[0180] b) Add concentrated sulfuric acid (mass fraction: 98%) and hydrogen peroxide (concentration: 30%) to slurrying liquid 1, wherein, the amount of hydrogen peroxide added is 1.2 times of the theoretical amount, and concentrated sulfuric acid i...
Embodiment 3
[0191] 1. Recycling and producing lithium carbonate from waste lithium iron phosphate batteries
[0192] a) Discharge, shred, and separate the waste lithium iron phosphate battery to obtain the casing, diaphragm, and positive and negative electrodes; roast the positive and negative electrodes at 450°C in a nitrogen atmosphere, and then pulverize and sieve to obtain copper aluminum and positive and negative electrode powders . Among them, copper and aluminum are separated into copper powder and aluminum powder through variable-diameter dry separation columns for recycling. The positive and negative electrode powders and washing water were mixed at a solid-to-liquid ratio of 1:1.5 for slurrying, and reacted for 10 minutes to obtain slurrying liquid 1.
[0193] b) Add concentrated sulfuric acid (mass fraction: 98%) and hydrogen peroxide (concentration: 30%) to slurry mixing liquid 1, wherein, the amount of hydrogen peroxide added is 2 times of the theoretical amount, and concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap