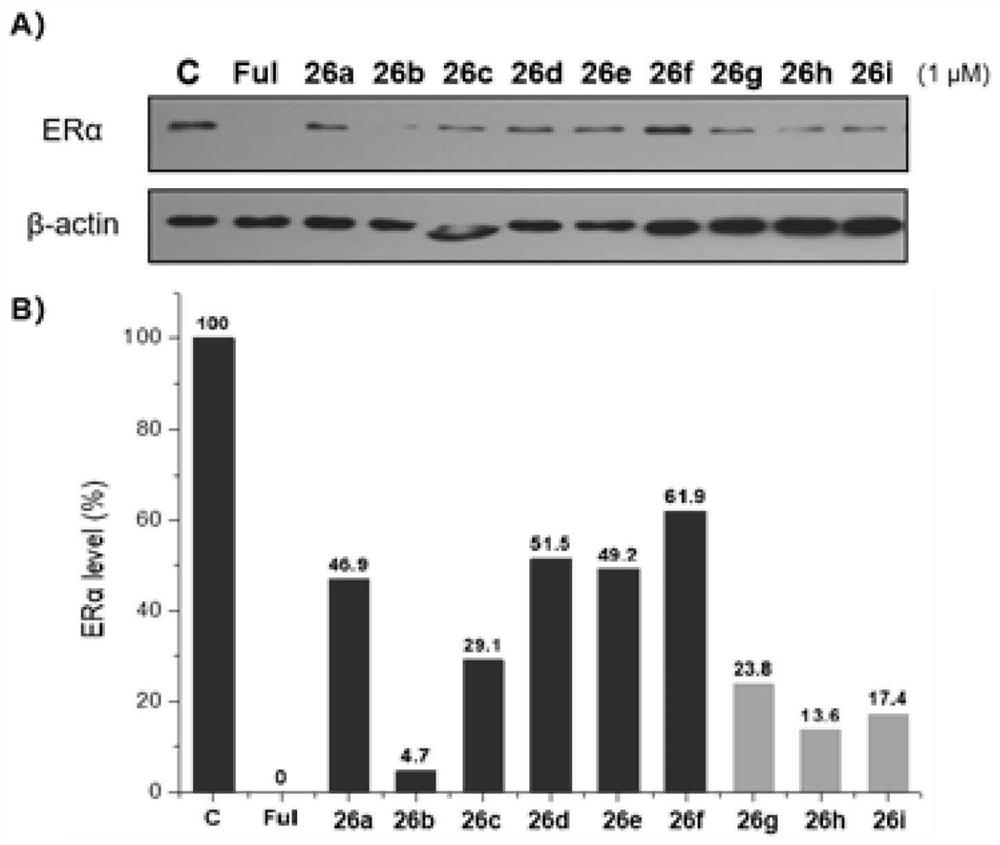
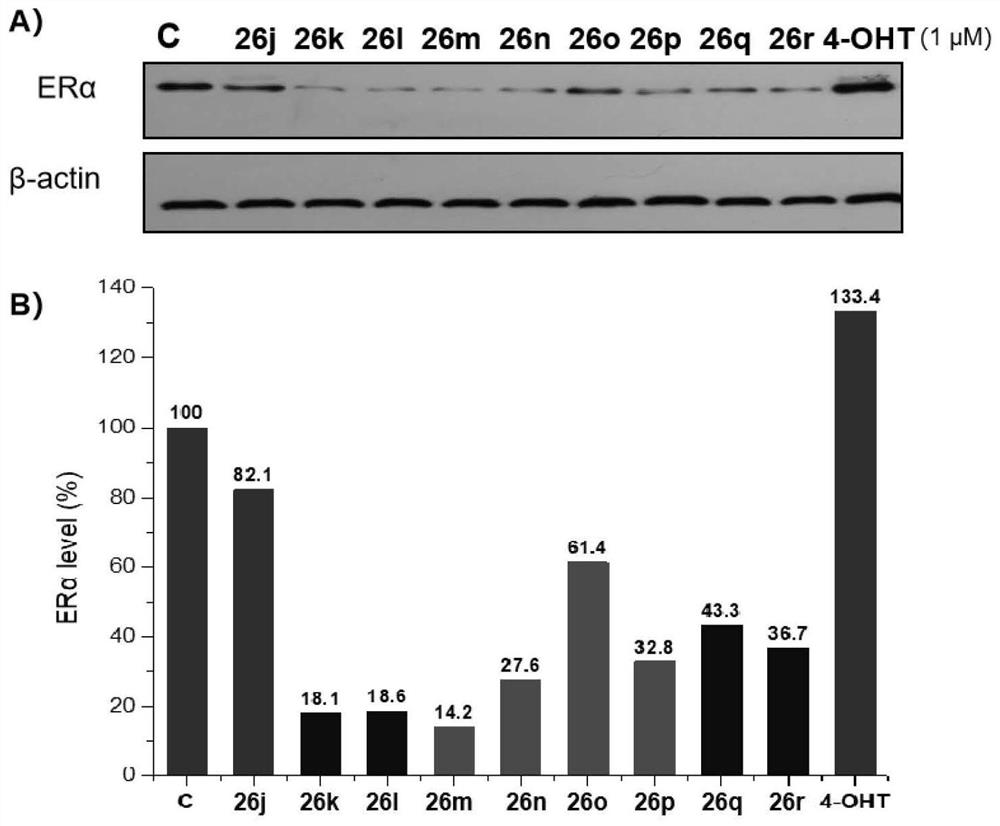
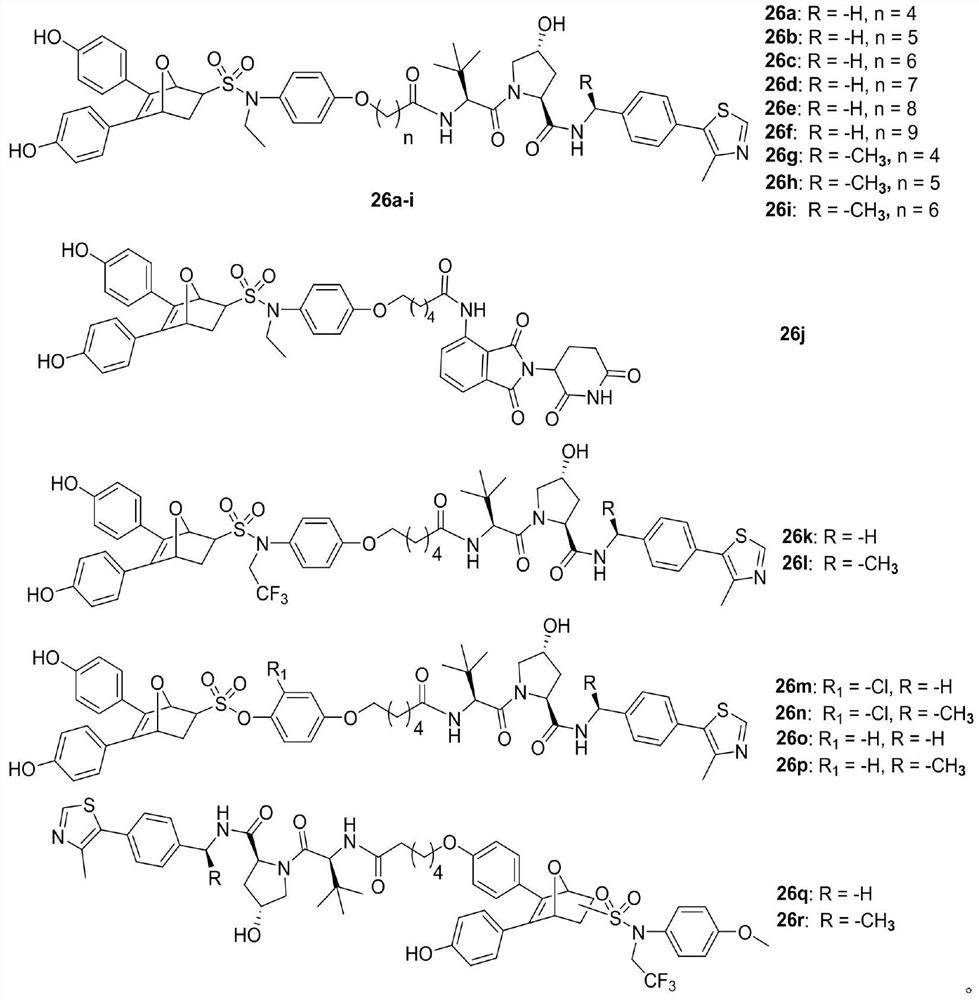
Proteolytic targeted chimeric compound taking oxygen bridge bicycloheptene compound as estrogen receptor ligand as well as preparation method and application
A technology of estrogen receptor and bicycloheptene, applied in the field of medicine, can solve the problems of triplet activity influence and difficulty in regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] (2S,2R)-1-((2S)-2-(5-(4-((N-ethyl-5,6-bis(4-hydroxyphenyl)-7-oxabicyclo[2.2.1 ]-5-heptene)-2-sulfonamido)phenoxy)pentanamide)-3,3-dimethylbutyryl)-4-hydroxy-N-(4-(4-methylthiazole-5 -yl)benzyl)pyrrolidine-2-carboxamide (26a)
[0090]
[0091] Under the protection of argon, bishydroxyphenylfuran 22 (35 mg, 0.14 mmol) and dienophile 25a (103 mg, 0.14 mmol) were placed in a round bottom flask, then anhydrous THF (2 mL) was added as a cosolvent . The reaction was stirred under heating at 90° C. for 8 hours. After the reaction was complete as detected by TLC, water was added to quench the reaction, and extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated, followed by silica gel column chromatography to obtain 87 mg of yellow solid product, 63% yield; m.p.172-173°C; 1 H NMR (400MHz,MeOD)δ8.86(s,1H),7.46(d,J=8.1Hz,2H),7.39(d,J=8.1Hz,2H), 7.12(dd,J=11.6,5.2Hz ,6H),6.82–6.74(m,4H),6.72–6.68(m,2H),5.4...
Embodiment 2
[0093] (2S,2R)-1-((2S)-2-(6-(4-((N-ethyl-5,6-bis(4-hydroxyphenyl)-7-oxabicyclo[2.2.1 ]-5-heptene)-2-sulfonamido)phenoxy)caproylamide)-3,3-dimethylbutyryl)-4-hydroxy-N-(4-(4-methylthiazole-5 -yl)benzyl)pyrrolidine-2-carboxamide (26b)
[0094]
[0095] Under the protection of argon, bishydroxyphenylfuran 22 (35 mg, 0.14 mmol) and dienophile 25b (106 mg, 0.14 mmol) were placed in a round bottom flask, then anhydrous THF (2 mL) was added as a cosolvent . The reaction was stirred under heating at 90° C. for 8 hours. After the reaction was complete as detected by TLC, water was added to quench the reaction, and extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated, followed by silica gel column chromatography to obtain 94 mg of yellow solid product, 67% yield; m.p.176-177°C; 1 H NMR (400MHz,MeOD)δ8.82(s,1H),7.43(d,J=8.1Hz,2H),7.35(d,J=8.1Hz,2H), 7.14–7.04(m,6H),6.74 (t,J=9.1Hz,4H),6.68(d,J=7.6Hz,2H),5.42(...
Embodiment 3
[0097] (2S,2R)-1-((2S)-2-(7-(4-((N-ethyl-5,6-bis(4-hydroxyphenyl)-7-oxabicyclo[2.2.1 ]-5-heptene)-2-sulfonamido)phenoxy)heptanamide)-3,3-dimethylbutyryl)-4-hydroxy-N-(4-(4-methylthiazole-5 -yl)benzyl)pyrrolidine-2-carboxamide (26c)
[0098]
[0099] Under the protection of argon, bishydroxyphenylfuran 22 (35 mg, 0.14 mmol) and dienophile 25c (107 mg, 0.14 mmol) were placed in a round bottom flask, then anhydrous THF (2 mL) was added as an auxiliary solvent. The reaction was stirred under heating at 90° C. for 8 hours. After the reaction was complete as detected by TLC, water was added to quench the reaction, and extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated, followed by silica gel column chromatography to obtain 91 mg of yellow solid product, 64% yield; m.p.178-179°C; 1H NMR (400MHz,MeOD)δ8.82(s,1H),7.43(d,J=8.2Hz,2H),7.36(d,J=8.1Hz,2H), 7.14–7.06(m,6H),6.79 –6.71(m,4H),6.68(d,J=8.6Hz,2H),5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com