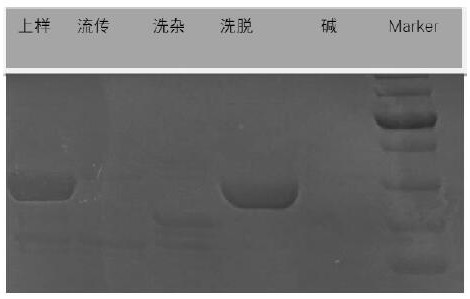
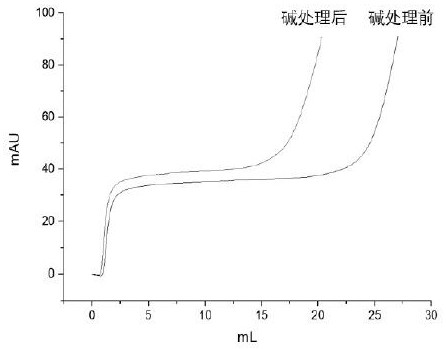
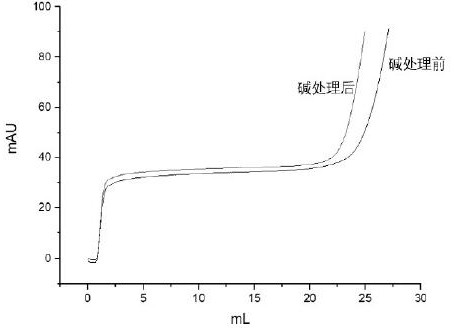
Immunoglobin conjugated protein and application thereof
A technology for immunoglobulins and binding proteins, which is applied in the direction of immunoglobulins, applications, and peptide preparation methods, and can solve problems such as high requirements for physical and chemical properties, unsatisfactory binding antibody loading, and poor tolerance of alkaline reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 protein multimer
[0060] Expression of engineering bacteria:
[0061] All the gene synthesis work in this experiment was entrusted to Nanjing GenScript to complete the expression strain E.coli BL (DE3).
[0062] The protein multimer prepared in this example is a six-segment repeat, and its monomer is obtained by splicing partial fragments of E-domain, C-domain and Z-domain:
[0063] E-domain
[0064] AQHDEAQQNA FYQVLNMPNL NADQRNGFIQ SLKDDP SQSA NVLGEAQKLN DSQAPK
[0065] D-domain
[0066] ADAQQNNFNKDQQSAFYEILNMPNLNEAQRNGFIQSLKDDPSQSTNVLGEAKKLNESQAPK
[0067] A-domain
[0068] ADNNFNKEQQNAFYEILNMPNLNEEQRNGFIQSLKDDPSQSANLLSEAKKLNESQAPK
[0069] B-domain
[0070] ADNKFNKEQQ NAFYEILHLP NLNEEQRNGF IQSLKDDPSQ SANLLAEAKK LNDAQAPK
[0071] C-domain
[0072] ADNKFNKEQQ NAFYEILHLP NLT EEQRNGF IQSLKDDPSV SKEILAEAKK LNDAQAPK
[0073] Z-domain
[0074] VDNKFNKEQQ NAFYEILHLP NLN EEQRNAF IQSLKDDP SQ SANLLAEAKK LNDAQAPK
[0075] The se...
Embodiment 2
[0140] The preparation of embodiment 2Protein A affinity packing
[0141] activation
[0142] 1. Accurately measure 300ml of 2mol / L NaOH solution, fully mix the solution with 200g of PMMA microspheres after draining and pour it into the reaction kettle, turn on the stirring motor and set the speed to 200rpm, set the temperature of the water bath to 40°C, and seal the reaction 30min.
[0143] 2. Accurately measure 125ml of dimethylformamide and 75ml of 1,4-butanediol glycidyl ether into the reaction kettle, seal and react for 2.5h.
[0144] 3. Take down the reactor, pour the microsphere mixture into a suction filter funnel to drain, and wash with 2CV deionized water. Then wash with 2CV alcohol and deionized water respectively, and drain until no water drips.
[0145] coupling
[0146] 1. Accurately weigh 10 g of activated microspheres and place them in a cleaned and dried Erlenmeyer flask.
[0147]2. Weigh 0.3g of the ligand and dissolve it fully with 12.8ml of buffer. Ad...
Embodiment 3
[0152] Example 3 Preparation of Protein A affinity filler
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com