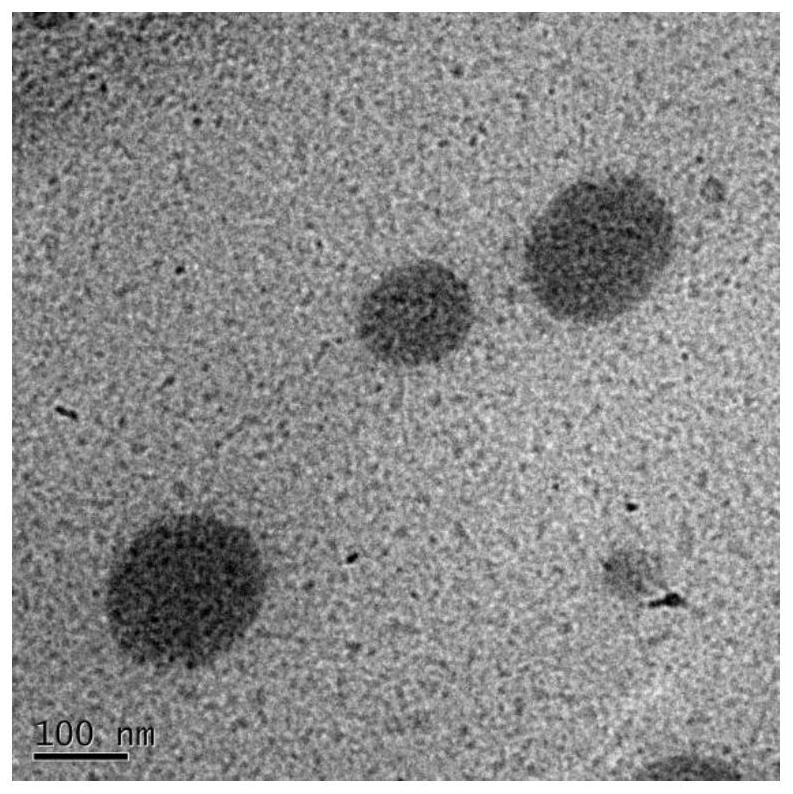
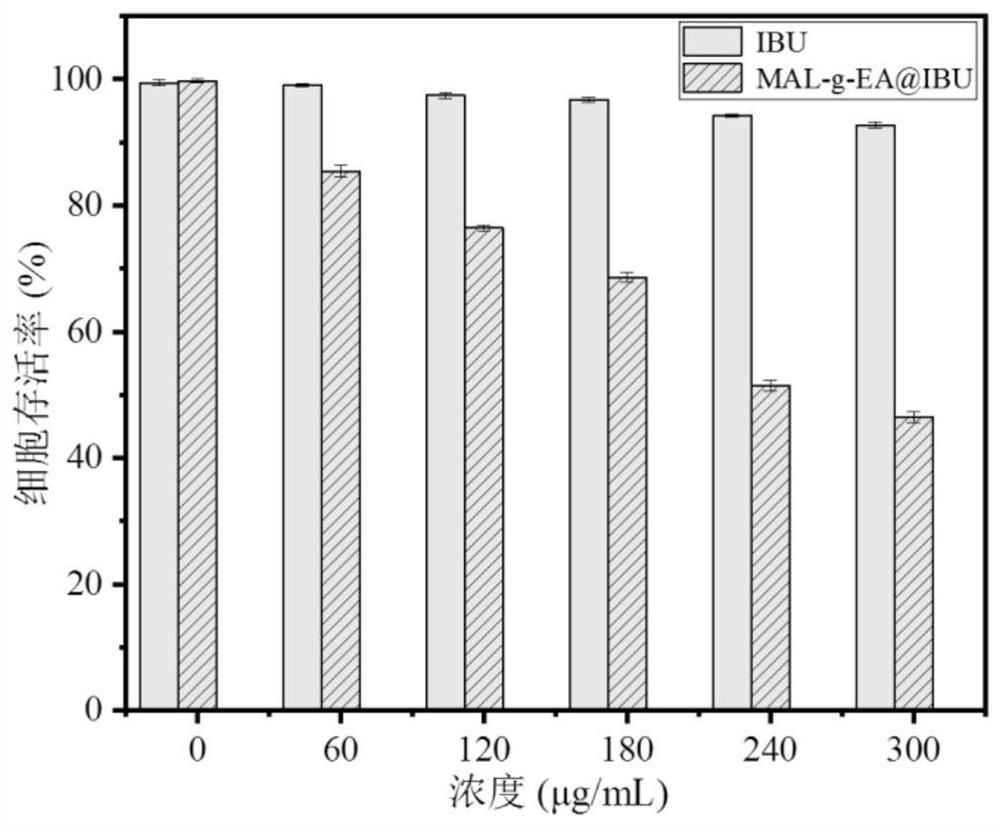
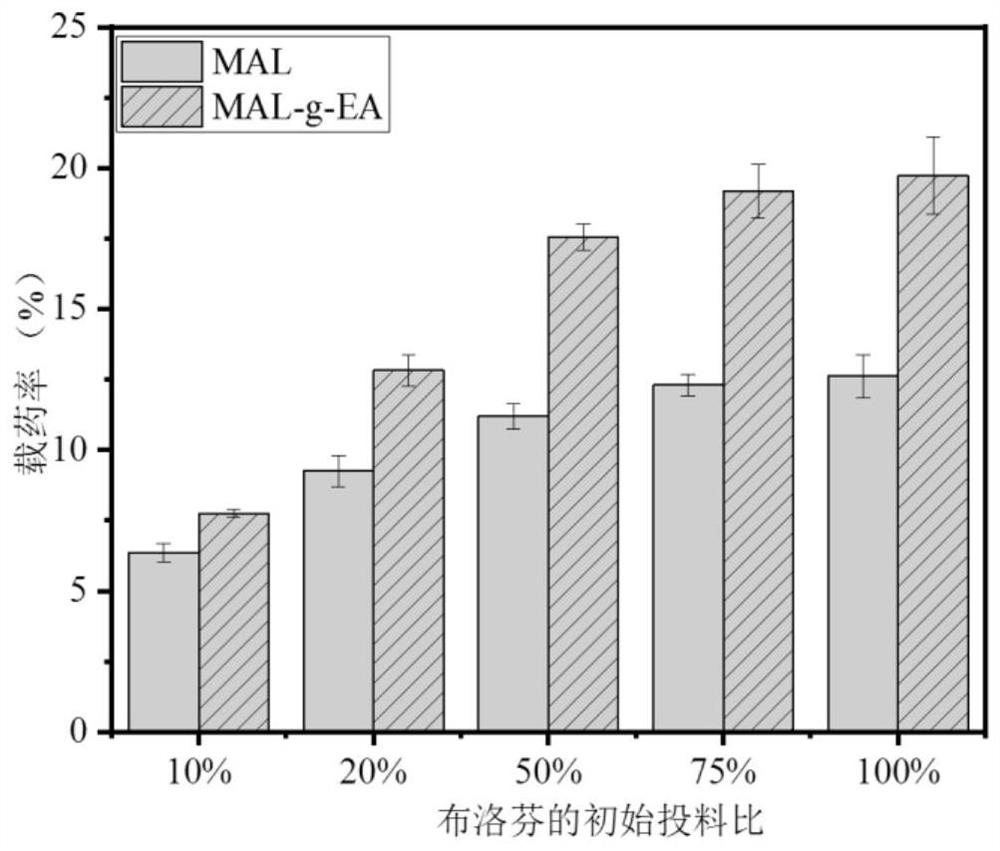
Multibiologically active modified lignin self-assembled drug-loaded nanomicelles and preparation method thereof
A biologically active and drug-loaded nanotechnology, which is applied in the direction of pharmaceutical formulations, organic active ingredients, and medical preparations of non-effective ingredients, can solve problems that have not been related to research, and achieve rich raw material sources, simple processes, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing multi-biologically active modified lignin self-assembled drug-loaded nanomicelles specifically includes the following steps:
[0037] Step 1, 2.00g of lignin, 0.49g (5.0mmol-CO 2 ) of maleic anhydride and 25 mg (0.2 mmol) of 4-dimethylaminopyridine (DMAP) were added to 30 mL of N,N-dimethylformamide (DMF) and reacted for 4 h under stirring in a constant temperature water bath at 50 °C. After the reaction, 300 mL of pure water was added to make it precipitate, filtered, washed three times with pure water, three times with absolute ethanol, and vacuum-dried at 50 °C to obtain acylated lignin acyl, namely maleylated lignin (MAL);
[0038]Step 2. Dissolve 2.0 g of MAL, 0.15 g (0.50 mmol) of ellagic acid (EA) and 3 mg (0.025 mmol) of 4-dimethylaminopyridine (DMAP) in 10 mL of DMF, and add it to a 100 mL round bottom flask After stirring for 5 min, 10 mL of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride dissolved in 114 mg (0.6 mmol) was s...
Embodiment 2-8
[0046] The parameter comparison table of embodiment 1 and embodiment 2-8 is shown in Table 1:
[0047] Among them: alkali lignin, enzymatic hydrolyzed lignin, sulfate lignin and kraft lignin are respectively represented by A1-A4; maleic anhydride, phthalic anhydride and succinic anhydride are represented by B1-B3 respectively; ellagic acid, white Veratrol and curcumin are represented by C1-C3, respectively.
[0048] The parameter comparison table of table 1 embodiment 1-8
[0049]
[0050] The process steps and other process parameters of the above-mentioned embodiments 2-8 are the same as those of the embodiment 1.
[0051] The drug loading rate data of Examples 1-8 are shown in Table 2.
[0052] Table 2 Example 1-8 product package drug loading rate table of ibuprofen (the initial charge of IBU is 50%)
[0053] serial number Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Example 7 Example 8 Drug loading rate (%) 17.55 15.637...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com