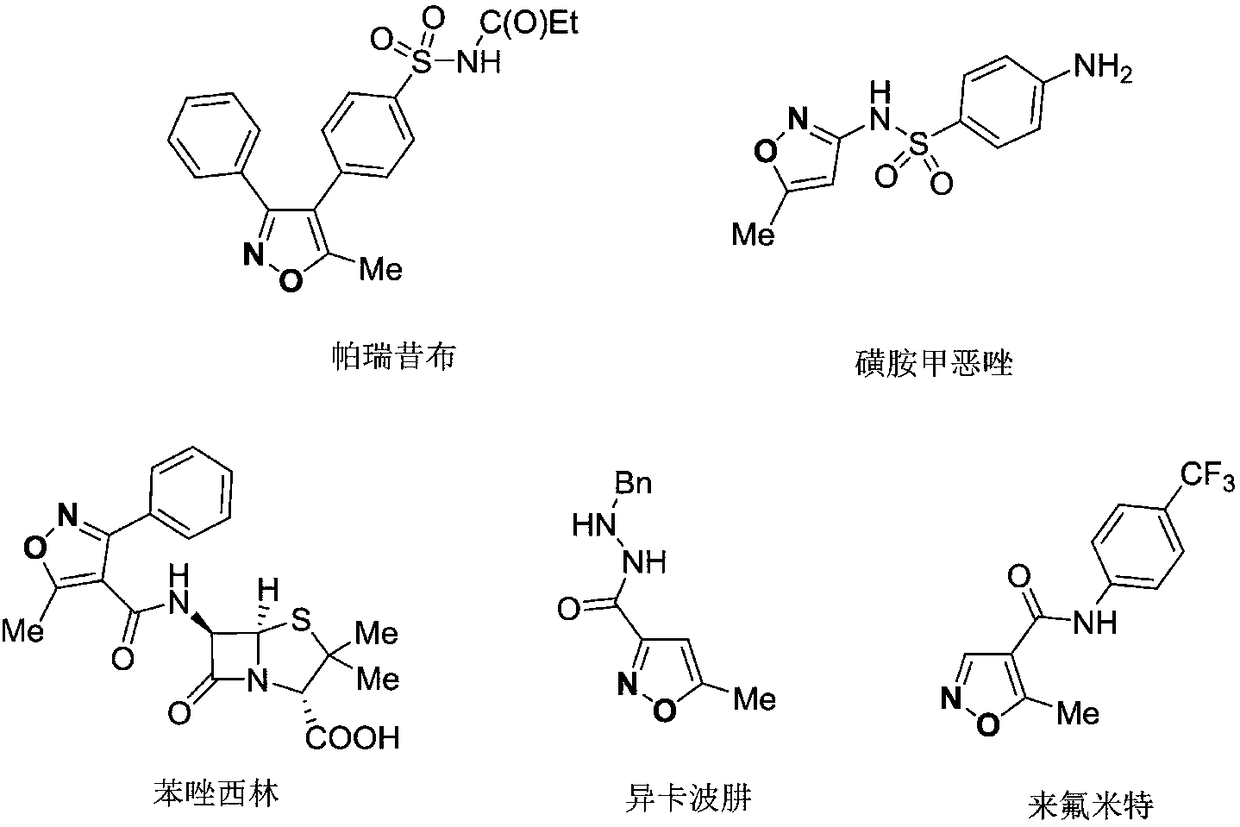
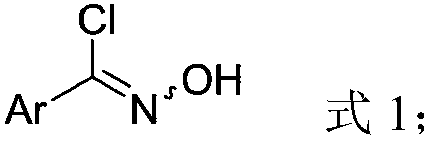Preparation method for 5-sulfuryl-fluoride-substitued isoxazoles compound
A technology for isoxazoles and compounds, which is applied in the field of preparation of 5-sulfonyl fluoride-substituted isoxazoles, can solve problems such as the lack of synthetic methods, and achieve the effects of simple and easy-to-obtain raw materials, mild reaction conditions, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
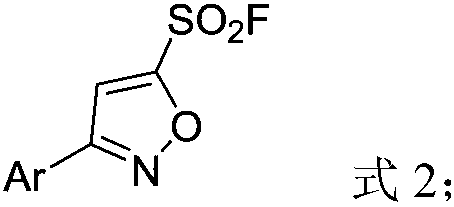
[0027] In a 50mL reaction flask, add phenyl chloroxime (0.78g, 5mmol), tert-butanol (10mL), tripropylamine (1.79g, 12.5mmol), stir at room temperature for 15min, then take 1-bromovinylsulfonyl Fluorine (3.78g, 20mmol) was dissolved in tert-butanol (10mL), placed in a 20mL constant pressure dropping funnel, and added dropwise to the above reaction system within 30min. The reaction process was detected by thin-layer chromatography. After the reaction, the reaction liquid was extracted with water and dichloromethane (3×10mL). The dichloromethane layer was dried with anhydrous sodium sulfate, filtered, and spin-dried. Analysis and purification (eluent is petroleum ether: ethyl acetate = 20:1 (v / v)), that is, 3-phenyl-5-sulfonyl fluoride isoxazole (885mg, 78%. M.p.69-70 ℃). 1 H NMR (500MHz,) δ7.83 (d, J=7.2Hz, 2H), 7.58–7.49 (m, 4H), 7.47 (s, 1H). 19 F NMR (471MHz, CDCl 3 )δ64.5(s,1F). 13 CNMR (126MHz,) δ163.3(s), 158.8(d, J=39.0Hz), 131.8(s), 129.6(s), 127.2(s), 12...
Embodiment 2
[0029]
[0030] In a 50mL reaction flask, add 4-benzyloxyphenyl chloroxime (1.31g, 5mmol), tert-butanol (10mL), tripropylamine (1.79g, 12.5mmol), stir at room temperature for 15min, then take 1-bromo Vinylsulfonyl fluoride (3.78g, 20mmol) was dissolved in tert-butanol (10mL), placed in a 20mL constant pressure dropping funnel, and added dropwise to the above reaction system within 30min. The reaction process was detected by thin-layer chromatography. After the reaction, the reaction liquid was extracted with water and dichloromethane (3×10mL). The dichloromethane layer was dried with anhydrous sodium sulfate, filtered, and spin-dried. Analysis and purification (eluent is sherwood oil: ethyl acetate=20:1 (v / v)), obtains 3-(4-benzyloxyphenyl)-5-sulfonyl fluoride isoxazole (1.33g , 80%.M.p.131-132°C). 1 H NMR (500MHz, CDCl 3 )δ7.76(d, J=8.4Hz, 2H), 7.47–7.39(m, 5H), 7.36(t, J=7.0Hz, 1H), 7.10(d, J=8.4Hz, 2H), 5.14( s,2H). 19 F NMR (471MHz, CDCl 3 )δ64.3(s,1F). 13 C NMR (...
Embodiment 3
[0032]
[0033] In a 50mL reaction flask, add 2-bromophenylchloroxime (1.17g, 5mmol), tert-butanol (10mL), tripropylamine (1.79g, 12.5mmol), stir at room temperature for 15min, then take 1-bromoethylene Sulfonyl fluoride (3.78g, 20mmol) was dissolved in tert-butanol (10mL), placed in a 20mL constant pressure dropping funnel, and added dropwise to the above reaction system within 30min. The reaction process was detected by thin-layer chromatography. After the reaction, the reaction liquid was extracted with water and dichloromethane (3×10mL). The dichloromethane layer was dried with anhydrous sodium sulfate, filtered, and spin-dried. Analysis and purification (eluent is petroleum ether: ethyl acetate = 20:1 (v / v)), to obtain 3-(2-bromophenyl)-5-sulfonyl fluoride isoxazole (793mg, 52% ). 1 H NMR (500MHz, CDCl 3)δ7.74(d, J=7.9Hz, 1H), 7.69(d, J=7.4Hz, 1H), 7.65(s, 1H), 7.48(t, J=7.4Hz, 1H), 7.42(t, J=7.3Hz,1H). 19 F NMR (471MHz, CDCl 3 )δ64.8(s,1F). 13 C NMR (126MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com