Immune cells expressing chimeric antigen receptor
A chimeric antigen receptor and cell technology, applied in the field of immunotherapy, can solve the unsatisfied problems of multiple myeloma treatment improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0344] 1. A chimeric antigen receptor (CAR) polypeptide comprising:
[0345] a) two or more extracellular domains each comprising a tumor necrosis factor (TNF) superfamily receptor ligand or a portion thereof;
[0346] b) a transmembrane domain; and
[0347] c) Intracellular signaling domain.
[0348] 2. The CAR polypeptide of paragraph 1, wherein the transmembrane domain comprises a hinge / transmembrane domain.
[0349] 3. The CAR polypeptide of paragraph 1 or 2, further comprising one or more co-stimulatory domains.
[0350] 4. The CAR polypeptide of any of paragraphs 1-3, wherein the TNF superfamily receptor ligand is a proliferation-inducing ligand (APRIL).
[0351] 5. The CAR polypeptide according to any one of paragraphs 1-3, wherein the TNF superfamily receptor ligand is TNF-α, lymphotoxin β, OX40 ligand (OX40L), CD154, Fas ligand (FasL ), LIGHT, TNF-like ligand 1A (TL1A), CD70, Siva, CD153, 4-1BB ligand (4-1BBL), TNF-related apoptosis-inducing ligand (TRAIL), nuclea...
Embodiment 1
[0518] Embodiment 1. Design of chimeric antigen receptor (CAR) based on APRIL
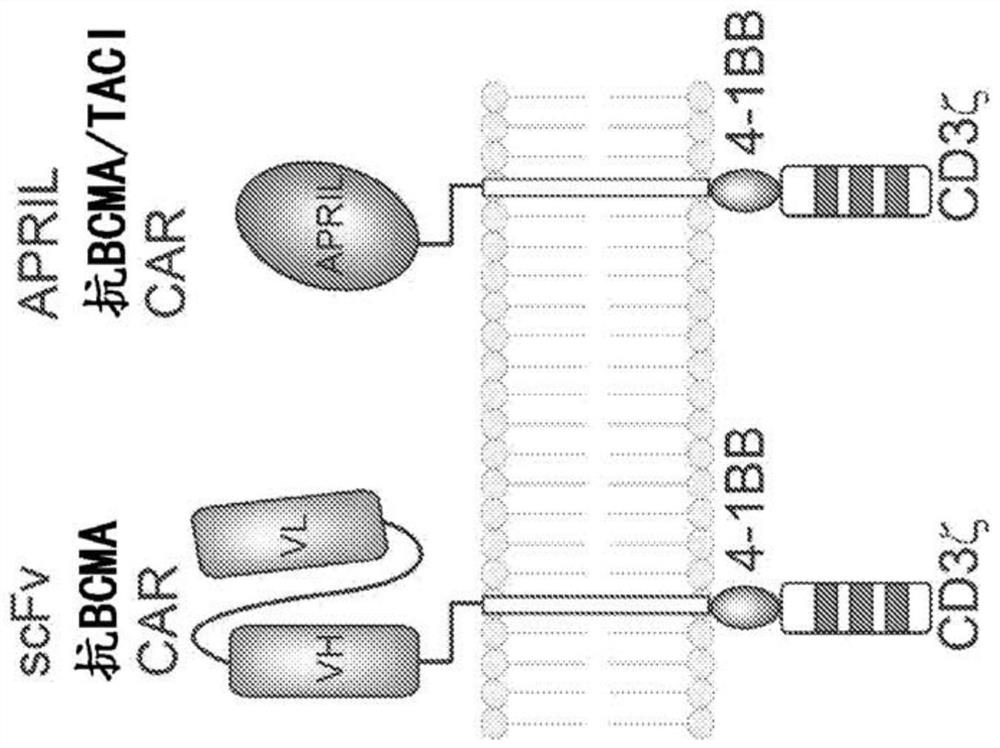
[0519]Described herein are chimeric antigen receptors, which are cells based on APRIL (proliferation-inducing ligand) fused to the transmembrane domain of CD8 or 4-1BB and the signaling domain of the T-cell activating receptors CD3ζ, CD3n, or CD3θ ectodomain. These CARs can overcome resistance to anti-BCMA-targeted therapies and utilize dimerization and trimerization transmembrane domains for optimal function. These CARs are considered for the treatment of cancer (eg, multiple myeloma), plasma cell disorders, and / or severe autoimmune diseases.
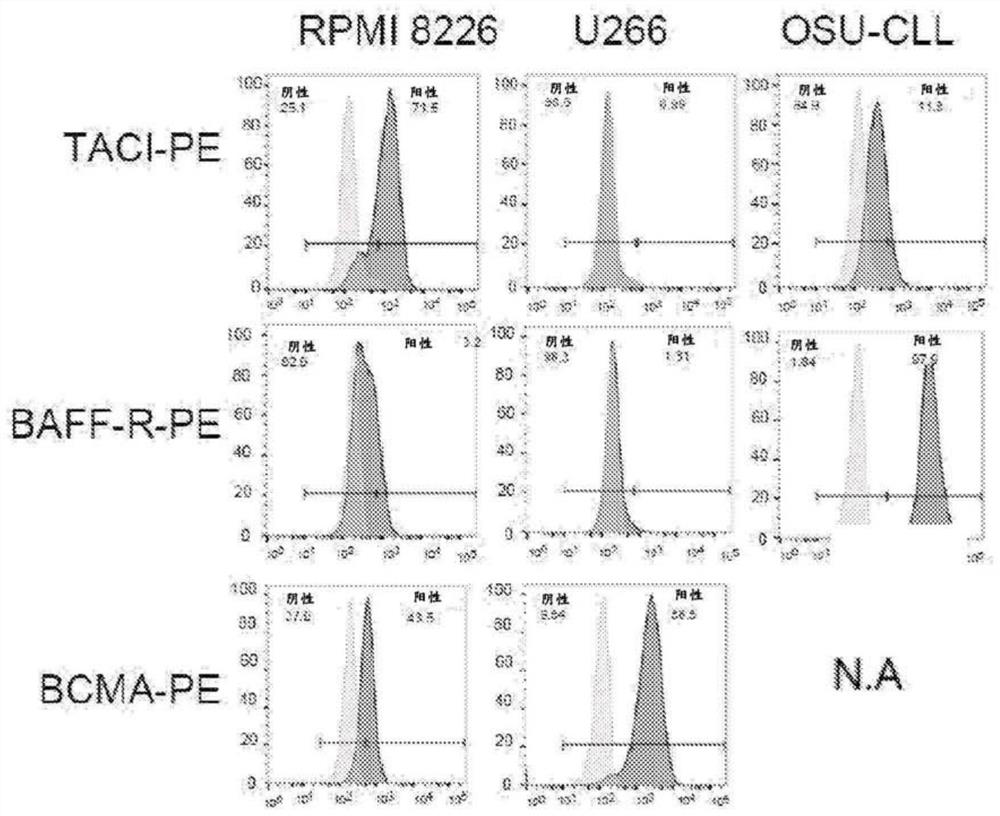
[0520] The inventors contemplate that natural ligands for BCMA can be used to engineer antigen-binding moieties to generate anti-myeloma CAR T cells. The cytotoxic activity, antigen-specific proliferation, and cytokine production of scFv-based and natural ligand (APRIL)-based CART cells in myeloma cell lines expressing BCMA, TACI, and / or BAFF receptors we...
Embodiment 2
[0535] Example 2. Limiting antigen escape in multiple myeloma by dual antigen targeting
[0536] Despite recent advances in treatment, multiple myeloma remains an incurable disease. Several recent clinical trials of CAR T cells targeting B-cell maturation antigen (BCMA) have resulted in clinical responses, including complete remissions in patients with multiple myeloma. However, treatment failure due to antigenic loss of BCMA has been described in some patients. The transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) is thought to have a redundant role for BCMA in maintaining plasma cell survival and is also highly expressed in multiple myeloma cells. In the work described here, APRIL, the natural ligand of BCMA and TACI, was used as the CAR binding moiety. The approach prevents disease relapse due to antigen escape by dual targeting multiple surface antigens in multiple myeloma ( image 3 ).
[0537] Materials and methods
[0538] CAR c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com