A polymer electrolyte based on surface initiation, its preparation and application
A polymer and electrolyte technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of low electrolyte conductivity, poor mechanical properties, poor cycle performance, etc., to achieve poor mechanical properties, good mechanical properties, large The effect of adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
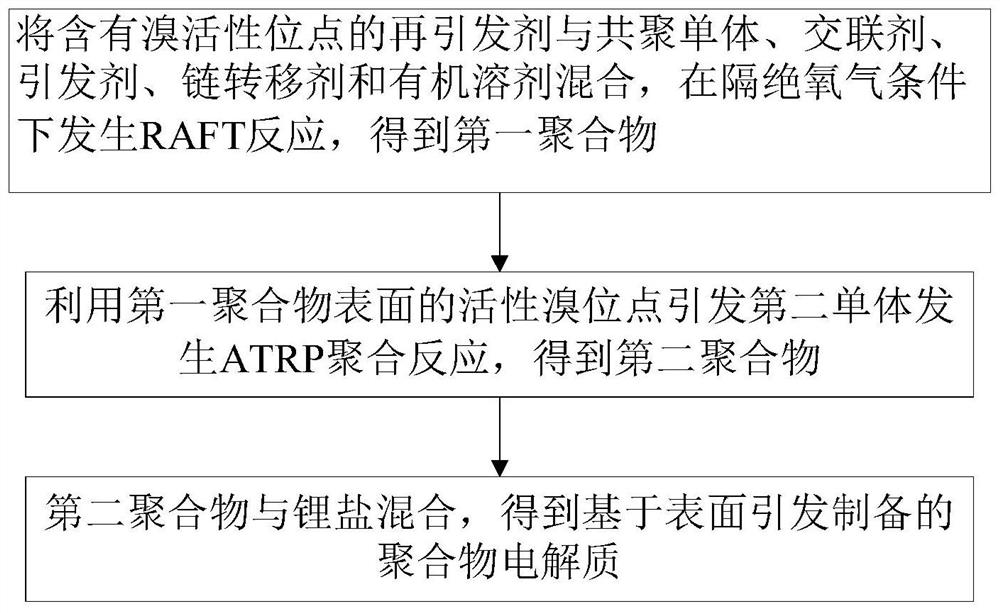
[0044] A kind of preparation method of polymer electrolyte provided by the invention, such as figure 1 shown, including the following steps:
[0045] (1) The first monomer is mixed with an acid bromide reagent containing a bromine initiation site, and an esterification reaction occurs under conditions in the presence of a solvent and an acid-binding agent, and separation and purification after the reaction obtains a re-initiator containing a bromine active site;
[0046] (2) Mix the re-initiator described in step (1) with comonomer, cross-linking agent, initiator, chain transfer agent and organic solvent, and reversible addition-fragmentation chain transfer polymerization (RAFT reaction) occurs under oxygen-free conditions ), to obtain the first polymer; the surface of the first polymer contains active bromine initiation sites;
[0047] (3) Mix the first polymer obtained in step (2), the solvent and the second monomer, and use the active bromine sites on the surface of the fi...
Embodiment 1
[0095] This example provides a method for preparing a polymer electrolyte prepared by surface reinitiation polymerization, and the preparation method is as follows:
[0096] S1: Add magneton, 1.21mL hydroxyethoxy methacrylate, 6.95mL triethylamine, and 80mL dichloromethane into a 250mL single-necked flask, keep it in an ice bath for 15 minutes, add 1.3mL bromoisobutyryl bromide, and stir 1h, the temperature was raised to 30°C and stirring was continued for 10h. The reaction solution was evaporated to remove the solvent, 80 mL of ethyl acetate was added, the filtrate was collected by suction filtration, 100 mL of saturated sodium bicarbonate solution was added to the filtrate, stirred for 2 hours, extracted, anhydrous magnesium sulfate was added to dry, and the solvent was removed by distillation under reduced pressure. Chromatography gave a tan liquid, which was the reinitiator 2-((2-bromo-2-methylpropionyl)oxy)ethyl methacrylate.
[0097]S2: Under anhydrous and oxygen-free c...
Embodiment 2
[0103] This example provides a method for preparing a polymer electrolyte prepared by surface reinitiation polymerization, and the preparation method is as follows:
[0104] S1: Add magnetite, 1.21mL hydroxyethoxy acrylate, 6.95mL diethylamine, and 80mL tetrahydrofuran into a 250mL single-necked flask, keep warm in an ice bath for 15 minutes, add 1.3mL bromopropionyl bromide, stir for 1 hour, and heat up to 15 ° C to continue stirring for 16h. The reaction solution was evaporated under pressure to remove the solvent, 80 mL of ethyl acetate was added, the filtrate was collected by suction filtration, 100 mL of saturated sodium bicarbonate solution was added to the filtrate, stirred for 2 h, extracted, anhydrous magnesium sulfate was added to dry, the solvent was removed by distillation under reduced pressure, and the column Chromatography gave a tan liquid, which was the reinitiator 2-((2-bromopropionyl)oxy)ethyl acrylate.
[0105] S2: Under anhydrous and oxygen-free condition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com