Separable and recyclable sulfide-type solid electrolyte and its application
A solid electrolyte, separation and recovery technology, applied in the field of materials, can solve the problems of extremely poor air stability, increase difficulty, increase cost, etc., achieve the effects of reducing polarization overpotential, high ion conductivity, and improving cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This example provides unmodified Li 4 SnS 4 The preparation process of the electrolyte material, which is also used as a comparison benchmark for the material properties of the separable and recoverable sulfide-type solid electrolyte proposed by the present invention in the subsequent embodiments.
[0050] This embodiment selects Li 2 S is Li source, SnS 2 As a Sn source, the air-stable (the water can be removed by heating after absorbing water after exposure to humid air / crystallization water to restore the original crystal structure) sulfide electrolyte Li by solid-phase method 4 SnS 4 ,Specific steps are as follows:
[0051] Will Li 2 S, SnS 2 The raw materials are weighed according to the design ratio and put into the ball milling jar, and the powder mass in each ball milling jar is 1g in total;
[0052] Two ball mill jars with raw materials are sealed, and placed in a planetary ball mill for ball milling for 40 hours;
[0053] After the ball milling is comp...
Embodiment 2
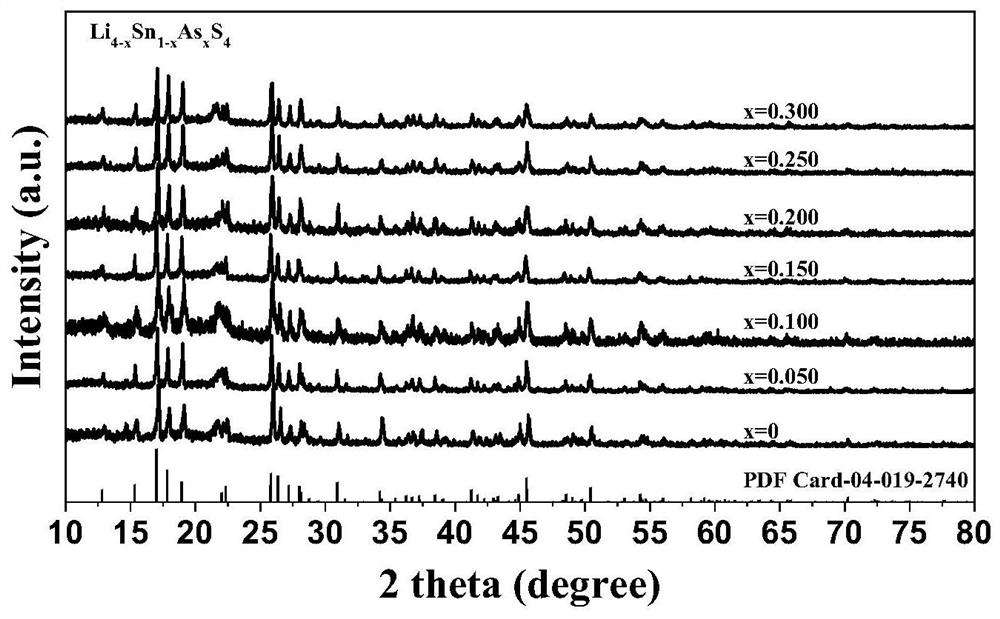
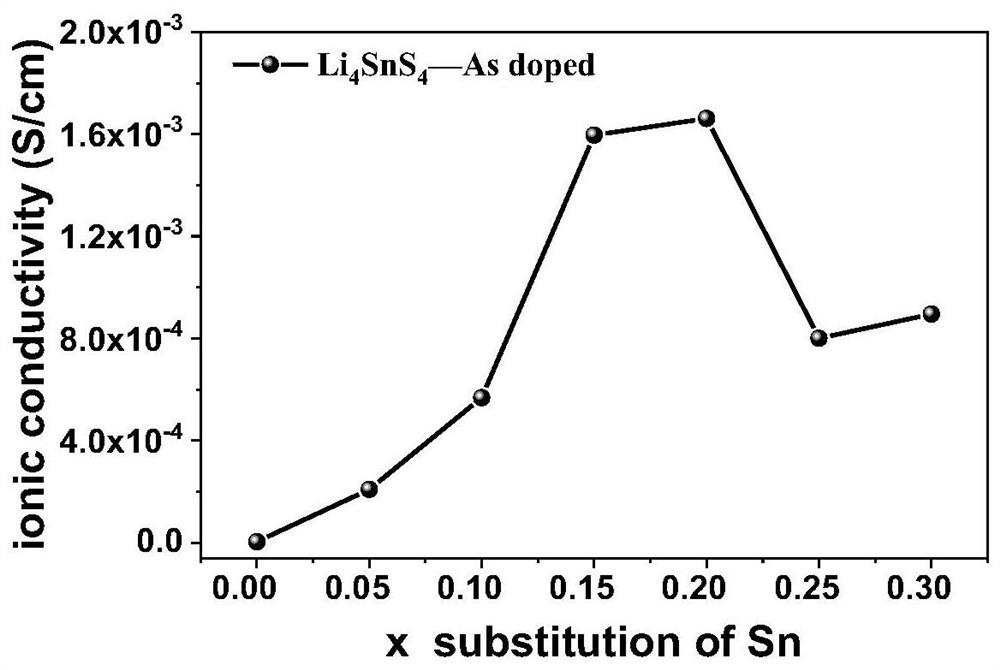
[0057] This example provides As-doped modified Li 4 SnS 4 Preparation process of electrolyte material.
[0058] In this embodiment, the commercially available and cheap Li 2 CO 3 is Li source, CS 2 as S source, SnO 2 is the Sn source, As 2 S 3 As a source of As, synthesize a sulfide electrolyte Li with high air stability (the water can be removed by heating / crystallization water to restore the original crystal structure after exposure to humid air and water absorption) and high ion conductivity 4-x sn 1-x As x S 4 (0
[0059] Will Li 2 CO 3 , SnO 2 、As 2 S 3 The raw materials were weighed according to the designed ratio and ground in a mortar for 30 min, and the total powder mass was 1 g, which was placed in an alumina crucible.
[0060] Add 50mL-100mL of CS 2 Liquid, add to a scrubber bottle with a capacity of 100mL.
[0061] Put the crucible filled with raw materials into the center of the quartz tube of the tube f...
Embodiment 3
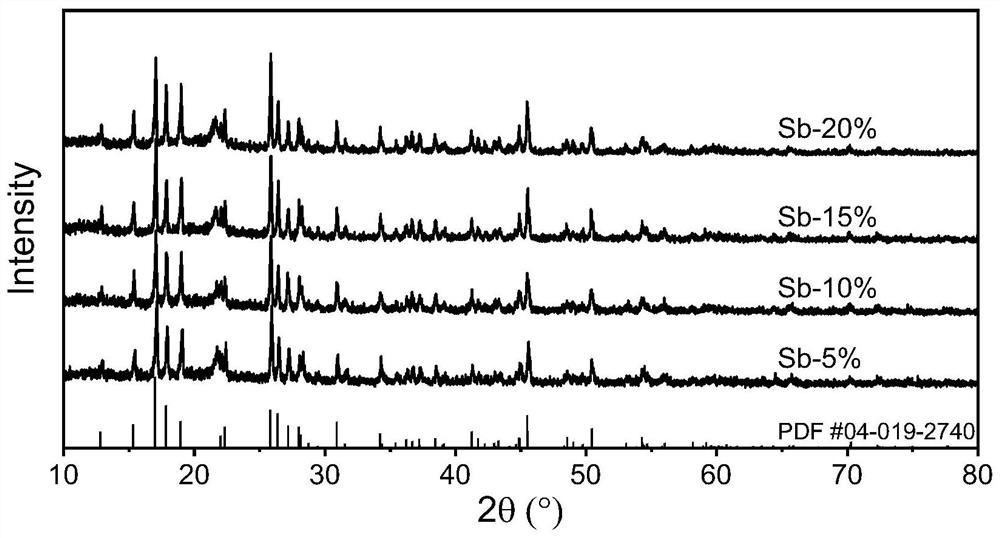
[0068] This example provides Sb-doped modified Li 4 SnS 4 Preparation process of electrolyte material.
[0069] In this embodiment, the commercially available and cheap Li 2 CO 3 is Li source, CS 2 as S source, SnO 2 is the Sn source, Sb 2 o 5 As a Sb source, synthesize a sulfide electrolyte Li with high air stability (the water can be removed by heating / crystallization water to restore the original crystal structure after exposure to humid air and water absorption) and high ion conductivity 4-x sn 1-x Sb x S 4 (0
[0070] Will Li 2 CO 3 , SnO 2 , Sb 2 o 5 The raw materials were weighed according to the designed ratio and ground in a mortar for 30 minutes, and the total powder mass was 1 g, which was placed in an alumina crucible.
[0071] Add 50mL-100mL of CS 2 Liquid, add to a scrubber bottle with a capacity of 100mL.
[0072] Put the crucible filled with raw materials into the center of the quartz tube of the tube...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com