Anti-human SIRP alpha monoclonal antibody and application thereof
A monoclonal antibody and antibody technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, applications, etc., can solve the problems of limited specificity of anti-SIRPα antibodies, negative effects on T cell proliferation and recruitment, and achieve novel sequences Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 obtains the mouse monoclonal antibody of specific anti-SIRPα by fusion hybridoma technology
[0042] 1.1 Animal immunity
[0043] Mice were immunized according to the general method in the literature (E Harlow, D. Lane, Antibody: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1998). 免疫原为C端含有人IgG1 Fc标签的重组人SIRPα(氨基酸Met 1-Arg 370)蛋白(公司自产,所用质粒为CD172-ECD-Fc;载体为pCP;插入氨基酸序列为MEPAGPAPGRLGPLLCLLLAASCAWSGVAGEEELQVIQPDKSVLVAAGETATLRCTATSLIPVGPIQWFRGAGPGRELIYNQKEGHFPRVTTVSDLTKRNNMDFSIRIGNITPADAGTYYCVKFRKGSPDDVEFKSGAGTELSVRAKPSAPVVSGPAARATPQHTVSFTCESHGFSPRDITLKWFKNGNELSDFQTNVDPVGESVSYSIHSTAKVVLTREDVHSQVICEVAHVTLQGDPLRGTANLSETIRVPPTLEVTQQPVRAENQVNVTCQVRKFYPQRLQLTWLENGNVSRTETASTVTENKDGTYNWMSWLLVNVSAHRDDVKLTCQVEHDGQPAVSKSHDLKVSAHPKEQGSNTAAENTGSNER)。 Use recombinant human SIRPα protein with his tag (produced by the company itself, the plasmid used is SRPA-ECD-His; the vector is pCMV-C-His; the amino acid sequence inserted between EcoR I an...
Embodiment 2
[0057] Example 2 In vitro analysis method for measuring the functional activity of SIRPα monoclonal antibody
[0058] 2.1 Determination of antibody binding ability based on capture ELSIA
[0059] Prepare goat anti-mouse IgG F(ab')2-specific secondary antibody (Jackson ImmunoResearch Laboratories, Inc., Cat#115-005-072) in 1xPBS to a final concentration of 2 μg / ml, add 100 μl / well to 96-well ELISA plates were coated at 37°C for 2 hours. After coating, wash the plate once with 1xPBST, add 200 μl / well of PBST solution containing 5% skimmed milk powder and place at 37 degrees Celsius for 2 hours to block. Wash the plate again, add 100 μl / well of diluted antibody solution and Benchmark (that is, KWAR23 antibody, produced by the company, and its heavy chain amino acid is
[0060] EVQLQQSGAELVKPGASVKLSCTASGFNIKDYYIHWVQQRTEQGLEWIGRIDPEDGETKYAPKFQDKATITADTSSNTAYLHLSSLTSEDTAVYYCARWGAYWGQGTLVTVSAASTKGPSVFPLAPCSRSTSESTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTKTYTCNVDHKPSN...
Embodiment 3
[0081] Example 3 Binding Activity of Anti-SIRPα Mouse Monoclonal Antibody
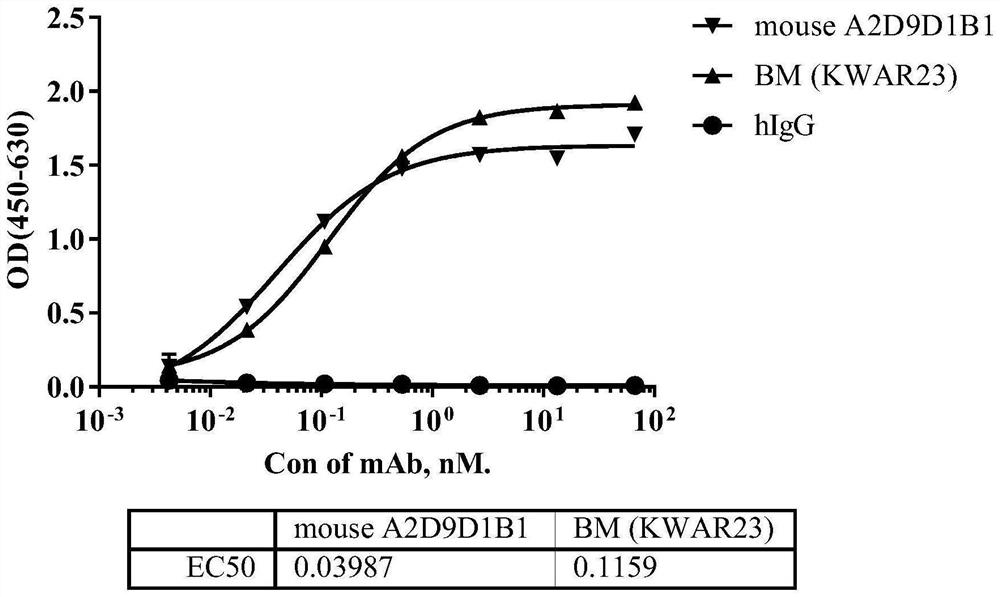
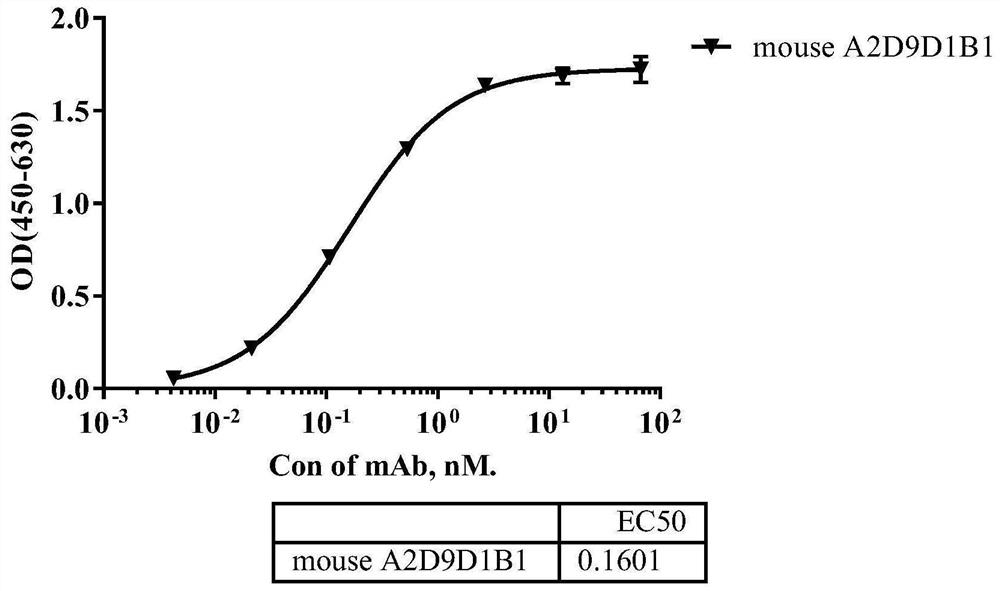
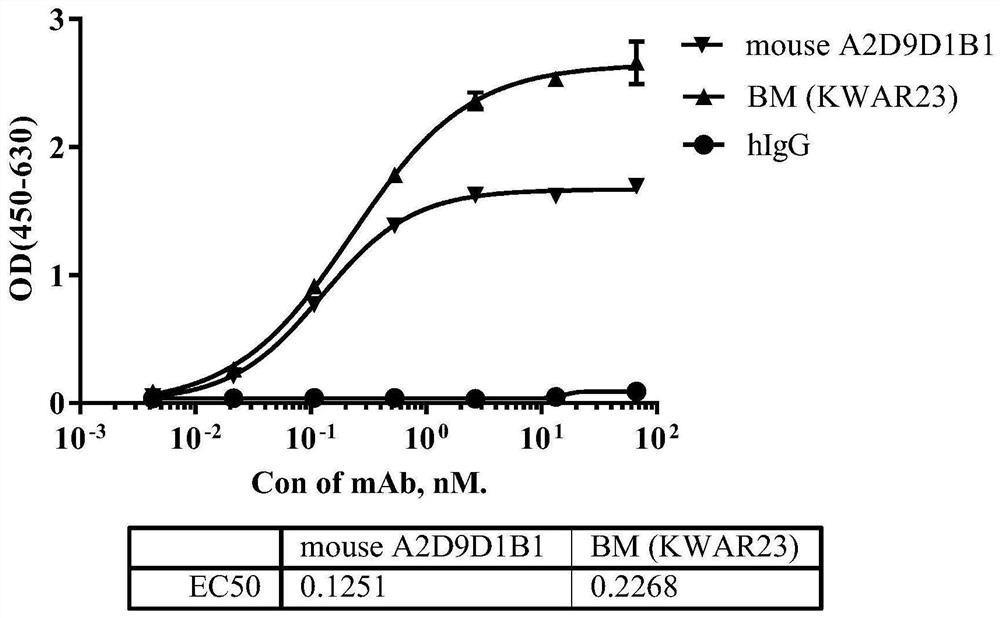
[0082] According to the analysis method described in Example 2, the evaluation of the binding activity of the SIRPα antibody is summarized in Table 3 below. Among them, SIRPα Benchmark was used as a control. It can be seen from Table 3 that the binding activity of the anti-human SIRPα monoclonal antibody of the present invention to human SIRPα antigen is slightly better than that of Benchmark, and can cross-react with SIRPβ antigen at the same time.
[0083] Table 3 Binding activity of SIRPα antibodies
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com