Cyclodextrin porous liquid material and application
A liquid material, cyclodextrin technology, applied in the direction of analyzing materials, material separation, instruments, etc., can solve the problems of small processing capacity and high cost, and achieve the effect of large processing capacity, low cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
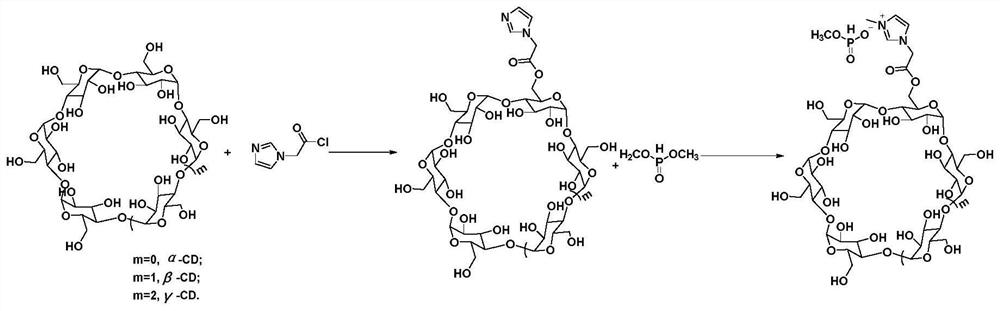
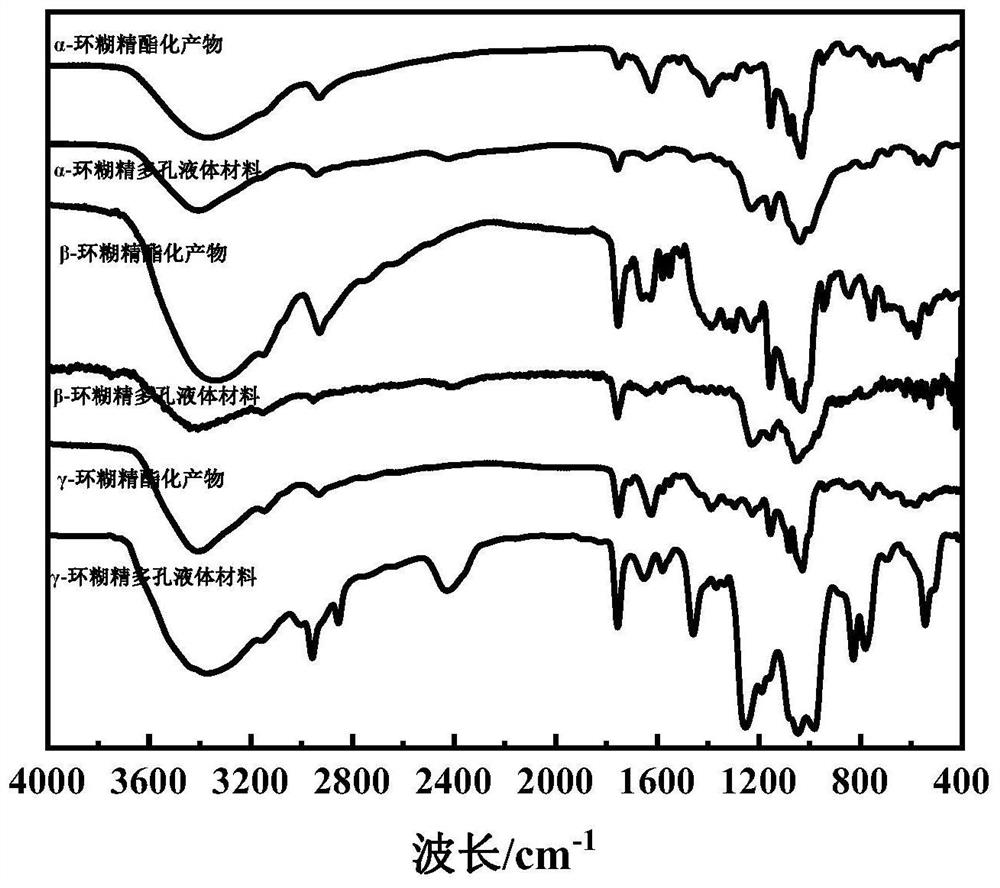
[0022] Will α -Cyclodextrin (1.2244 g, 1.26 mmol), imidazole-1-acetyl chloride (1.3542 g, 9.37 mmol) and 30 mL N,N-dimethylformamide were placed in a round bottom flask, and then triethylamine (0.9777 g, 9.66 mmol) was added to a round bottom flask, stirred and reacted at 0°C, and the reaction time was 24 h to obtain a reaction mixture, which was filtered with suction to obtain a filtrate, which was added dropwise to 100 mL of acetone, and filtered with suction to obtain a solid precipitate The solid precipitate was dried at 80°C for 4 hours to obtain 0.6685 g of α-cyclodextrin esterification product with a yield of 49.18%. will get α - The cyclodextrin esterification product (0.6685 g, 0.62 mmol) was mixed with dimethyl phosphite (1.0921 g, 9.92 mmol), and reacted under a microwave with a power of 40W to obtain a liquid, which was dried at 80°C for 4 hours to obtain α - Cyclodextrin porous liquid material 0.7124 g, yield: 96.71%.
Embodiment 2
[0024] Will β -Cyclodextrin (2.6158 g, 2.30 mmol), imidazole-1-acetyl chloride (2.6363 g, 18.24 mmol) and 30 mL of N,N-dimethylformamide were placed in a round bottom flask, and then triethylamine (1.4723 g, 14.55 mmol) was added to a round bottom flask, stirred and reacted at 12°C, and the reaction time was 28 h to obtain a reaction mixture, which was filtered with suction to obtain a filtrate, which was added dropwise to 100 mL of acetone, and filtered with suction to obtain a solid Precipitate, the solid precipitate was dried at 80°C for 4h to obtain β - Cyclodextrin esterification product 1.9704 g, yield: 68.82%. will get β - The cyclodextrin esterification product (1.9704 g, 1.59 mmol) was mixed with dibutyl phosphite (7.7215 g, 39.76 mmol), and reacted under a microwave with a power of 200 W to obtain a liquid, which was dried at 80°C for 4 hours, get β - Cyclodextrin porous liquid material 1.8569 g, yield: 87.86%.
Embodiment 3
[0026] Will gamma -Cyclodextrin (1.4911 g, 1.15 mmol), imidazole-1-acetyl chloride (1.5052 g, 10.41 mmol) and 30 mL of N,N-dimethylformamide were placed in a round-bottomed flask, and triethylamine (1.0567 g, 10.44 mmol) was added to a round-bottomed flask, stirred and reacted at 25°C, and the reaction time was 36 h to obtain a reaction mixture, which was filtered with suction to obtain a filtrate, which was added dropwise to 100 mL of acetone, and filtered with suction to obtain a solid Precipitate, the solid precipitate was dried at 80°C for 4h to obtain gamma - Cyclodextrin esterification product 0.7922 g, yield: 49.07%. will get gamma - The cyclodextrin esterification product (0.7922 g, 0.56 mmol) was mixed with dioctyl phosphite (5.2014 g, 17.00 mmol), and reacted under a microwave with a power of 40 W to obtain a liquid, which was dried at 80°C for 4 hours, get gamma - Cyclodextrin porous liquid material 0.7415 g, yield: 76.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com