Application of thymosin or derivative thereof and medicine for treating pleasant sensation deficiency type depression
A technology of anhedonia and thymosin, applied in the field of pharmacy, can solve the problems of acute poisoning, disordered balance relationship, anhedonia, etc., achieve good curative effect, improve anhedonia-type depression symptoms, and have no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0024] 1. Experimental animals
[0025] Sexually mature female C57BL / 6 mice, body weight (20-22g), were purchased from Shandong Experimental Animal Center (animal quality certificate: No. 37009200018914, license number: SCXK (Lu) 2014000). Breeding environment: 4 mice per cage, 22±1°C temperature, 50±10% humidity, 12h light-dark cycle, free to eat and drink. The feeding and operation of experimental animals complied with the relevant regulations on the feeding and use of experimental animals of Guangdong Ocean University.
[0026] Thymosin β4: purchased from Guangzhou Binshang Biotechnology Co., Ltd.
[0027] Rats and mice maintained powder feed: provided by Guangdong Experimental Animal Center.
[0028] 2. Experimental plan
[0029] After the animals were repurchased and adapted to the laboratory for one week, they were randomly numbered and grouped into 3 groups, as shown in Table 1:
[0030] Table 1. Grouping of experimental animals
[0031]
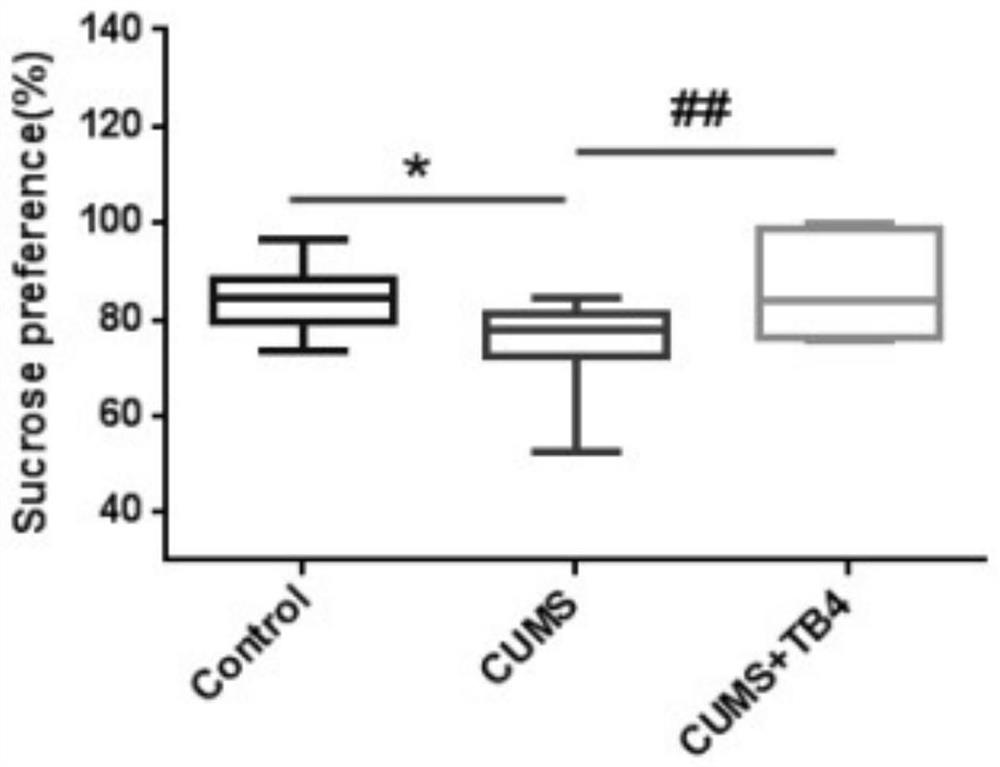
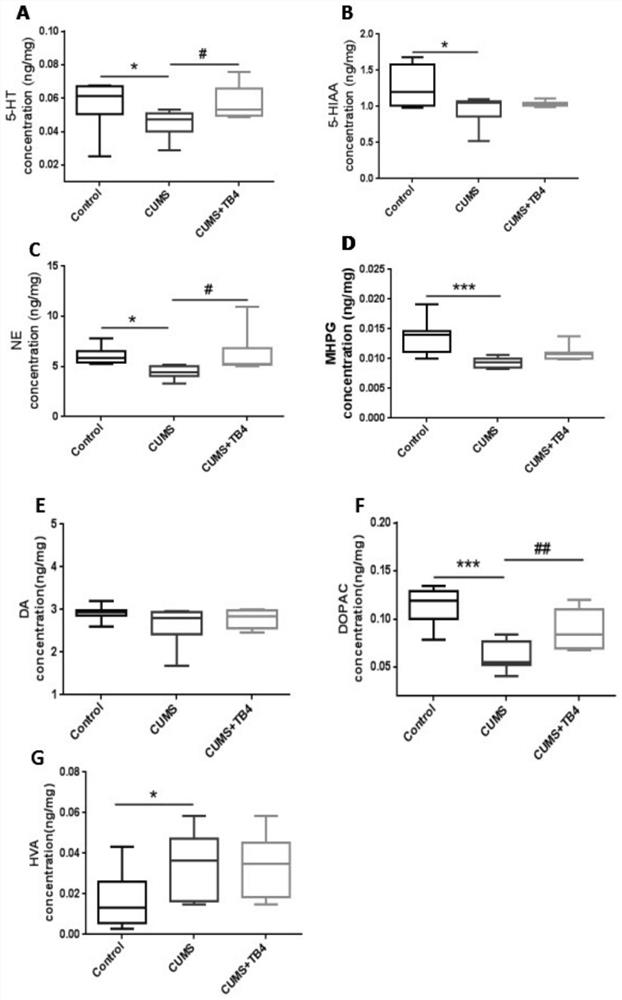
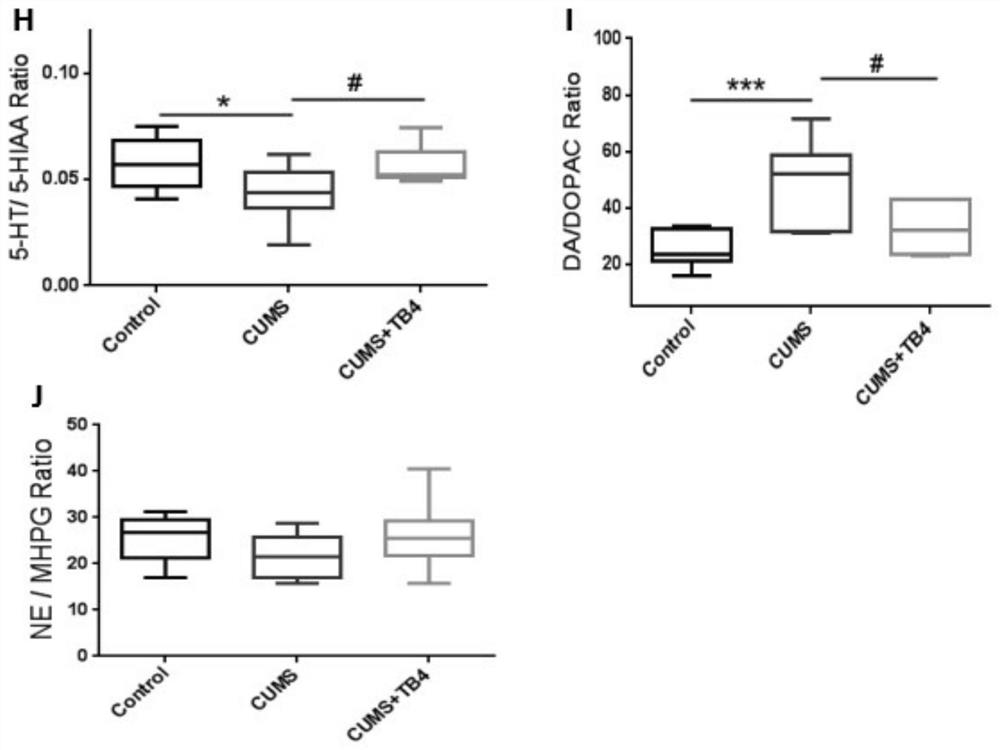
[0032] The CUMS group an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com