Method for preparing clinical-grade mesenchymal stem cell preparation by utilizing human induced pluripotent stem cells
A technology of pluripotent stem cells and stem cells, applied in artificially induced pluripotent cells, biochemical equipment and methods, animal cells, etc., can solve the problems of low homogeneity, unstable quality and limited sources, and reach the market The effect of broad prospects, high homogeneity and unlimited sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for preparing a clinical grade mesenchymal stem cell preparation using human induced pluripotent stem cells, comprising the steps of:
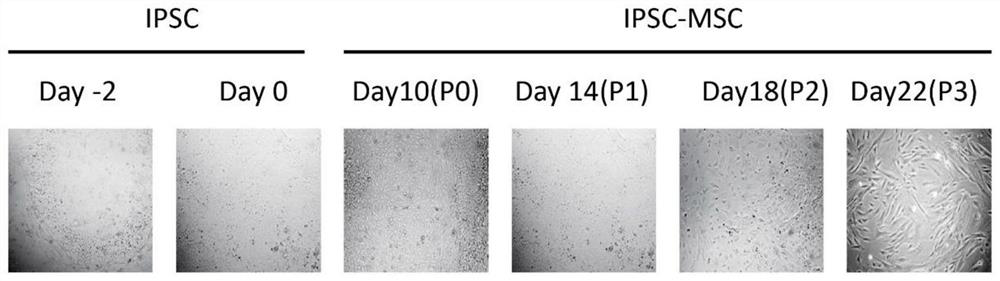
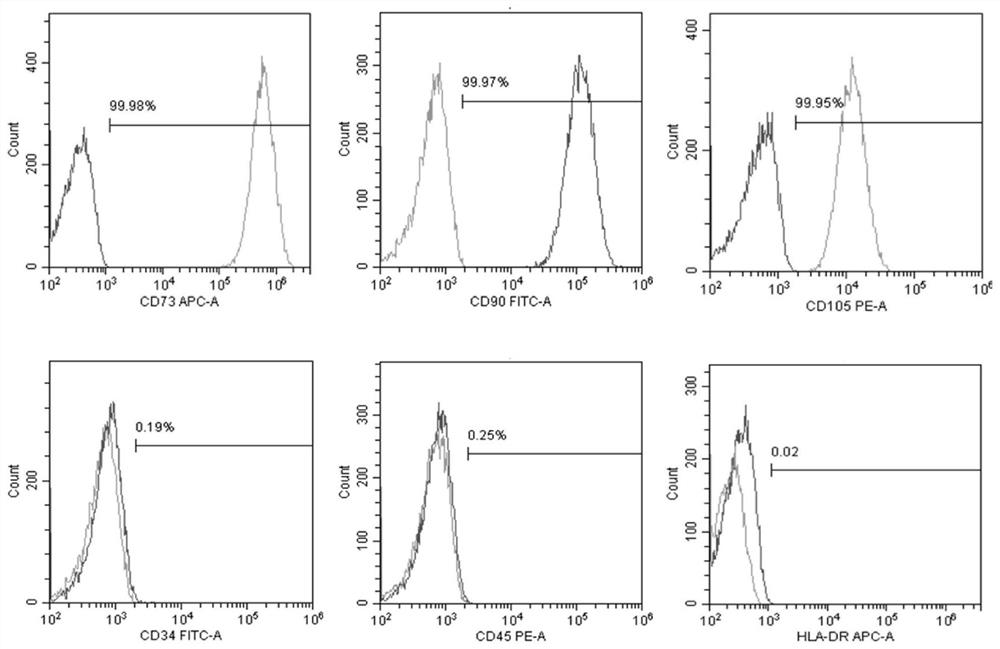
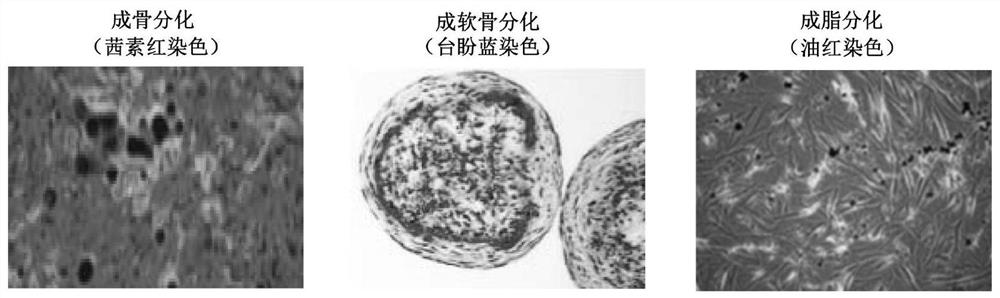
[0051] 1. Conventional culture of human iPSC: the human iPSC cell line was purchased from Nuwacell Company, which is a NuwacellTM scientific research grade hiPSC cell line (RC01001-B), which was inoculated in 10 μg / ml Vitronectin XF TM (composition includes 960μl CellAdhere TM DilutionBuffer+40μl 250μg / ml Vitronectin XF TM ) coated 6-well plate, using iPSC regular medium (1920μl TeSR TM -E8 TM Basal Medium+80μl 25×TeSR TM -E8 TM Supplement) at 37°C, 5% CO 2 Cultivate for 6 days under saturated humidity conditions. The above conventional medium was replaced with fresh one every day to continue culturing. During this period, the morphology of human iPSCs at different culture times was photographed with an optical microscope. see results figure 1. Undifferentiated human iPSCs were grown in 6-well plates on Day-2 before d...
Embodiment 2
[0060] A method for preparing a clinical grade mesenchymal stem cell preparation using human induced pluripotent stem cells, comprising the steps of:
[0061] 1. Routine culture of human iPSC: Human iPSC cell line (purchased from Nuwacell) was inoculated in 10 μg / ml Vitronectin XF TM (Consists of 960μl CellAdhere TM DilutionBuffer+40μl 250μg / mlVitronectin XF TM ) coated 6-well plate, using iPSC regular medium (1920μl TeSR TM -E8 TM Basal Medium+80μl 25×TeSR TM -E8 TM Supplement) at 37°C, 5% CO 2 Cultivate for 6 days under saturated humidity conditions. The above conventional medium was replaced with fresh one every day to continue culturing. During this period, the morphology of human iPSCs at different culture times was photographed with an optical microscope. see results figure 1 . Undifferentiated human iPSCs were grown in 6-well plates on Day-2 before differentiation to a 70% compact cobblestone-like multicellular colony with a high nucleoplasmic ratio and promin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com