A kind of binaphthyl organic polymer hole transport material and its synthesis method and application
A technology of hole transport material and synthesis method, which is applied in the field of binaphthyl organic polymer hole transport materials and its synthesis, and can solve the problems of unfavorable perovskite battery device stability, reduced material hole mobility, and molecular stacking. Low density and other issues, to achieve the effect of being conducive to commercial production and application, improving hole mobility, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
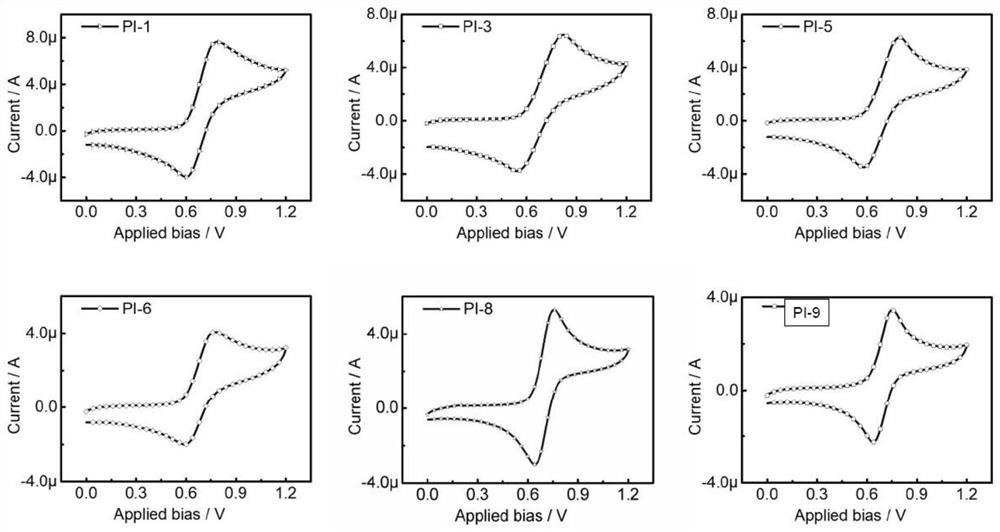
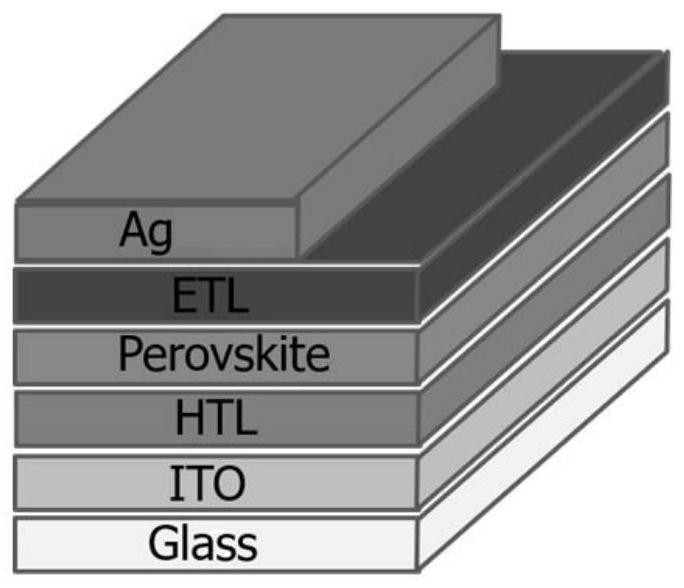
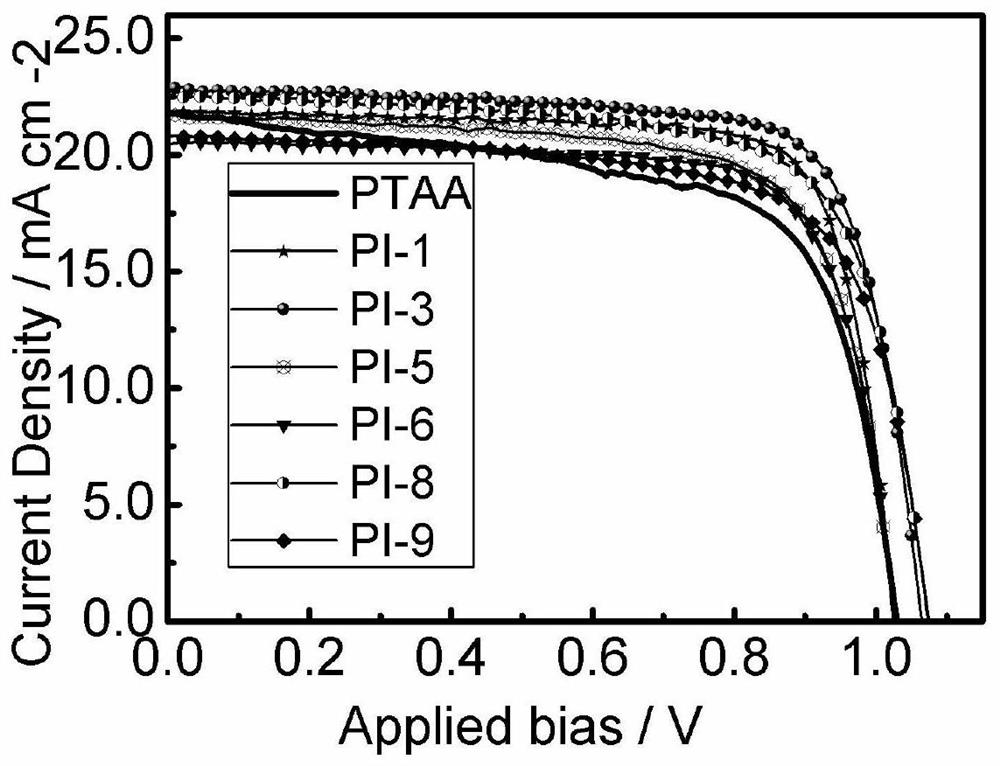
Image
Examples
Embodiment 1
[0039] Preparation of 6,6'-bis(4-(bis(4-(4-methoxyphenyl)amino)phenyl)-2,2'-binaphthol
[0040]
[0041] 6,6'-dibromo-2,2'-binaphthol 15 (8.71g, 19.61mmol), 4-boronic acid pinacol ester-4',4'-dimethoxytriphenylamine 16 (19.5 g, 45.21mmol) was dissolved in tetrahydrofuran, an aqueous solution of sodium carbonate (6.24g, 58.87mmol, 30ml) was added for nitrogen replacement under stirring, tetrakis(triphenylphosphine)palladium (680mg, 0.588mmol) was added, and the temperature was slowly raised to reflux; The reaction was about 8 hours, and the reaction was monitored by TLC. After the reaction, add water to quench the reaction, extract, and recrystallize the obtained crude product with petroleum ether and ethyl acetate to obtain 11.5 g of the final product, with a yield of 65.7%
[0042] 1 H NMR (400MHz, DMSO) δ9.28(s, 2H), 8.06(s, 2H), 7.90(d, J=8.9Hz, 2H), 7.56(d, J=8.5Hz, 4H), 7.45(t ,J=15.2Hz,2H),7.33(d,J=8.9Hz,2H),7.16–6.95(m,11H),6.89(m,12H),5.77(s,1H),3.74(s,12H) .
...
Embodiment 2
[0045] Preparation of Polymer PI-1
[0046]
[0047] 6,6'-bis(4-(bis(4-(4-methoxyphenyl)amino)phenyl)-2,2'-binaphthol Z5 (400mg, 0.45mmol), 1,2 -Dibromoethane (85mg, 0.45mmol) and sodium carbonate (192mg, 1.81mmol) were added to 5ml of dry N,N-dimethylformamide, stirred at 100°C for 24h, TLC monitored the completion of the reaction of the raw materials, and cooled the reaction system to room temperature , filtered, the filtrate was dropped in absolute ethanol under stirring, stirred, suction filtered, and the filter cake was dried under reduced pressure to obtain 250 mg of a yellow product with a yield of 59%.
Embodiment 3
[0049] Preparation of polymer PI-3
[0050]
[0051] 6,6'-bis(4-(bis(4-(4-methoxyphenyl)amino)phenyl)-2,2'-binaphthol Z5 (400mg, 0.45mmol), 1,6 - Add dibromohexane (111mg, 0.45mmol) and sodium carbonate (192mg, 1.81mmol) into 5ml of dry N,N-dimethylformamide, screw the bottle tightly, and stir at 100°C for 24h, TLC monitors the completion of the reaction of raw materials , the reaction system was cooled to room temperature, filtered, the filtrate was dropped into absolute ethanol under stirring, stirred, suction filtered, the filter cake was taken and dried under reduced pressure to obtain 220 mg of off-white solid powder with a yield of 50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com