Preparation method of piperacypress and cefoxil capsule
A technology for piperacillide and capsules, which is applied in the field of preparation of piperacillin capsules, can solve problems such as poor dissolution uniformity, and achieve the effects of improved content uniformity, good appearance and guaranteed product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Effect of Large Sheet Density
[0038] Since the dry granulation process first compresses large tablets and then granulates, different large tablet densities will affect the particle size distribution of the granules, and then affect the dissolution results. Therefore, the influence of different large tablet densities on the particle size distribution should be investigated.
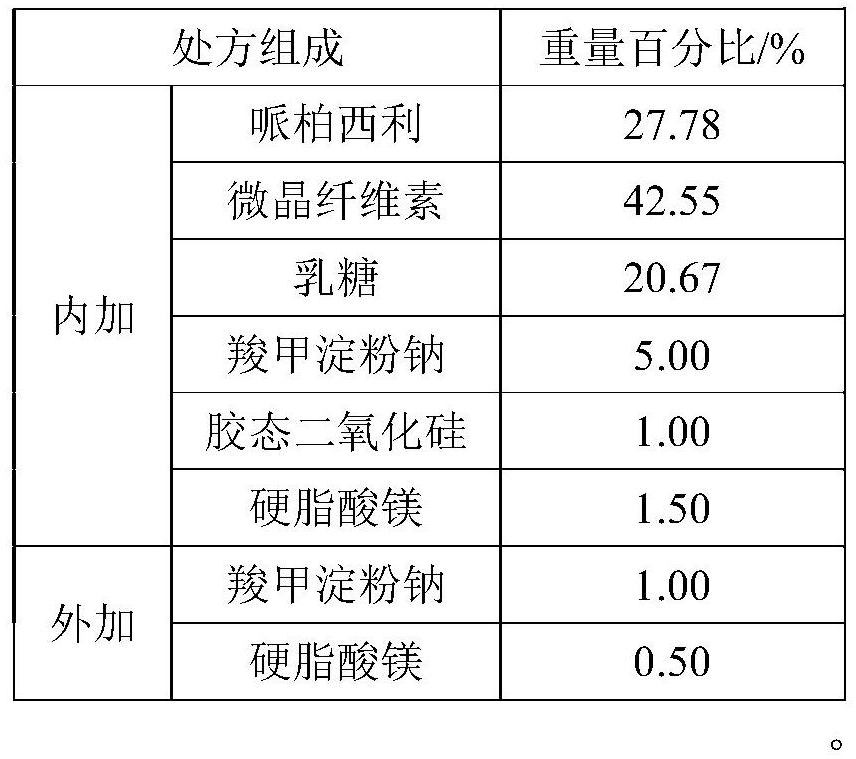
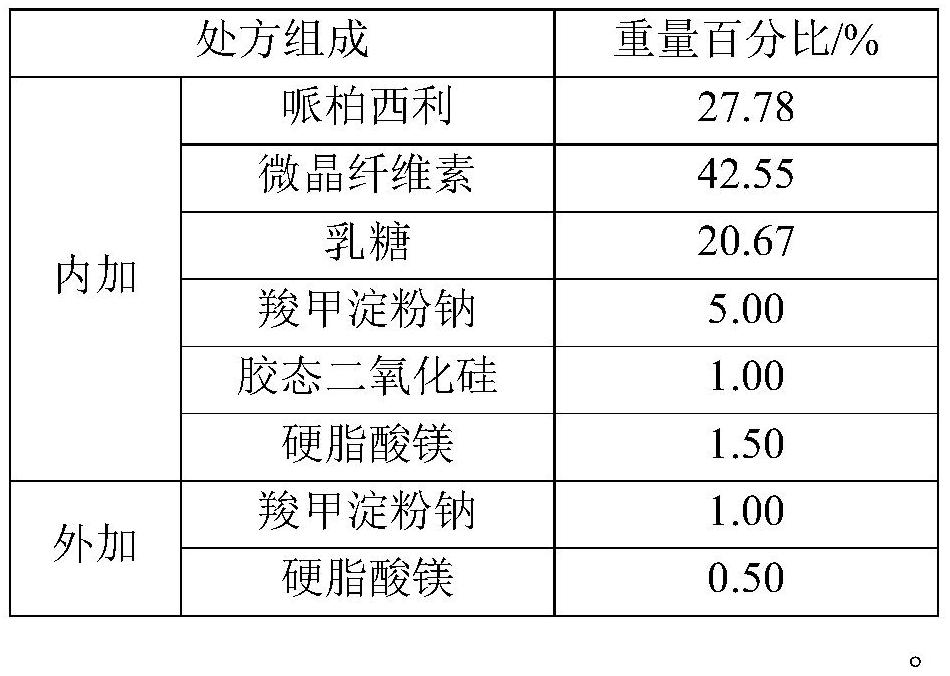
[0039] Table 1 Prescribing Information
[0040]
[0041]
[0042] Concrete preparation process is as follows:
[0043] (1) Premix:
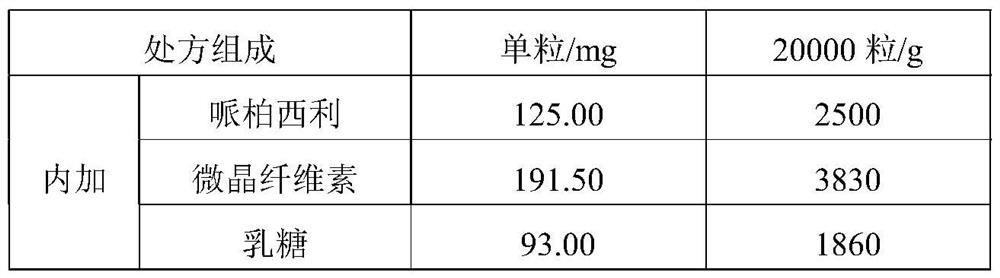
[0044] Premix 1: Weigh the prescription amount of palbociclib, microcrystalline cellulose, lactose, sodium starch glycolate (internal addition), colloidal silicon dioxide, magnesium stearate (internal addition), and mix for 2 minutes (speed: 10rpm ).
[0045] Pre-mixing 2: Pass the above-mentioned mixed powder through a sieve with a pore size of 1.0mm for granulation and deagglomeration, and then mix for 5 minutes (speed: 10rpm).
[0046] (2) Dry...
Embodiment 2
[0057] Example 2: Effects of different large flake densities on particle size and proportion of fine powder <60 mesh obtained by multiple granulation
[0058] Adopt the prescription of embodiment 1, control large sheet density 0.9-1.0g / cm 3 , and according to the following preparation method:
[0059] (1) Premix:
[0060] Premix 1: Weigh the prescription amount of palbociclib, microcrystalline cellulose, lactose, sodium starch glycolate (internal addition), colloidal silicon dioxide, magnesium stearate (internal addition), and mix for 2 minutes (speed: 10rpm ).
[0061]Pre-mixing 2: Pass the above-mentioned mixed powder through a sieve with a pore size of 1.0mm for granulation and deagglomeration, and then mix for 5 minutes (speed: 10rpm).
[0062] (2) Dry granulation (multiple granulation): Control the density of large flakes by adjusting the screw speed, drum speed and drum pressure. Pass the large pieces through a 20-mesh sieve for granulation and then sieve the granule...
Embodiment 3
[0072] Embodiment 3: the influence of different fine powder proportions on mixing uniformity
[0073] It is 1.0g / cm to choose that the bulk density is 1.0g / cm among the embodiment 2 3 or 0.9g / cm 3 The total mixed granule that step (3) makes at the same time. Samples were taken at different locations (11 sampling points in total) to determine the content uniformity of the blended particles.
[0074] Table 6 The density of large pieces is 1.0g / cm 3 , the impact of the particle size distribution of the obtained particles in step (2) on the content uniformity of the blended particles in step (3)
[0075]
[0076] Table 7 The density of large pieces is 0.9g / cm 3 , the impact of the particle size distribution of the obtained particles in step (2) on the content uniformity of the blended particles in step (3)
[0077]
[0078] According to the results of Table 6 and Table 7, it can be seen that when the bulk density is 1.0g / cm 3 or 0.9g / cm 3 Under normal circumstances, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com