Modeling method of mouse model for chronic contact dermatitis
A contact dermatitis and mouse model technology, applied in the field of biomedicine, can solve inappropriate problems and achieve the effect of a good screening platform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Fifteen 7-week-old female Balb / c mice were selected. Except for the normal control group (3 mice), the other 12 mice received 30 μL of 1.3% oxazolone in acetone-olive oil solution on the front and back of the right ear as the first trial. For sensitization challenge, the day was designated as day 0. Seven days later, the right ear of all mice received again 30 μL of 0.8% oxazolone in acetone-olive oil solution as a second sensitization challenge. Thereafter, 20 μL of 0.3-0.5% mass fraction of oxazolone in acetone-olive oil solution was used for sensitization challenge every 2-3 days. Specifically, 0.5%, 0.5%, 0.5%, and 0.3%. On the 17th day, 12 mice with chronic contact dermatitis were obtained.
Embodiment 2
[0034]Four 6-week-old female Balb / c mice were selected to receive 30 μL of 1.2% oxazolone in acetone and olive oil solution on the front and back of the right ear as the first sensitization challenge, and this day was designated as day 0. Seven days later, the right ear of all mice received again 30 μL of 0.9% oxazolone in acetone-olive oil solution as a second sensitization challenge. Thereafter, 20 μL of 0.4-0.6% mass fraction of oxazolone in acetone-olive oil solution was used for sensitization challenge every 2-3 days. Specifically, 0.6% and 0.4% , 0.4%. Four mice with chronic contact dermatitis were obtained.
Embodiment 3
[0035] Example 3 Mouse Model Verification
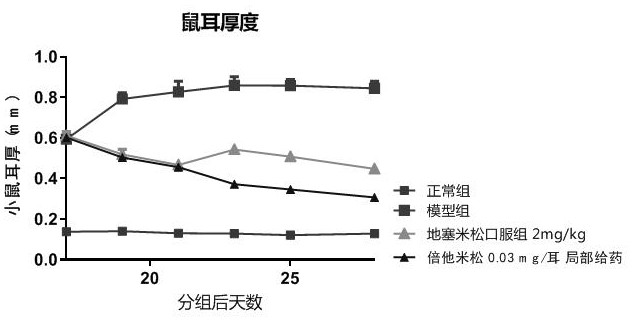
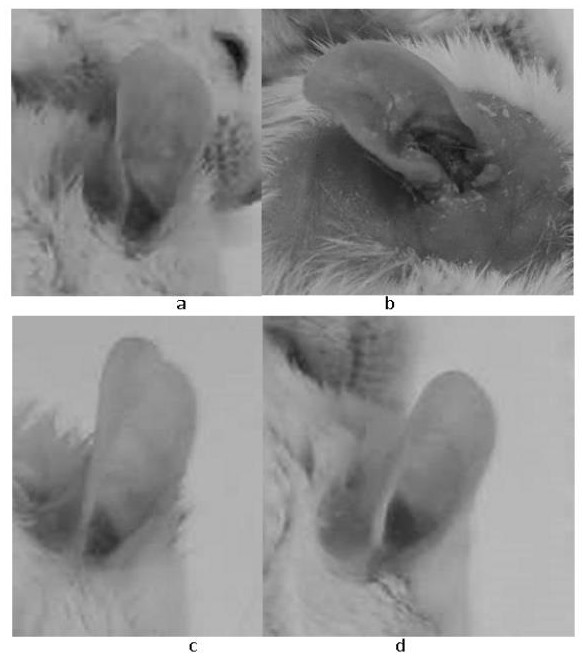
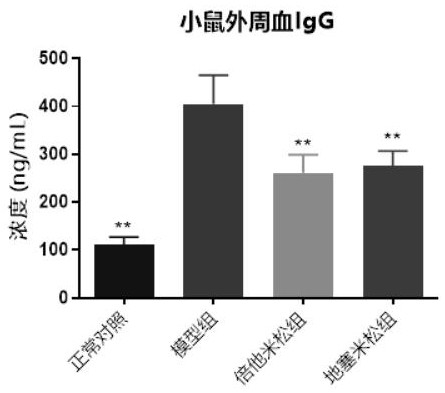
[0036] Three days after the last sensitization challenge in Example 1, that is, on the 17th day, the 12 chronic contact dermatitis mice obtained were grouped according to the thickness of the right ear, and 9 mice with similar thickness of the right ear were selected and divided into three groups ( 3 animals in each group, the average thickness of the right ear is basically the same), two groups of them began to administer treatment on the same day, and one group used the positive control drug betamethasone, and the administration method was 0.03mg / ear topical administration, which was dissolved in In acetone, smear on the front and back of the right ear of the mice in the positive drug group; the other group uses dexamethasone, and the administration method is 2 mg / kg orally. Because contact dermatitis is currently being treated clinically, topical steroids are mainly used for symptom control, so in this example, the common topical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com