Anti-tumor pharmaceutical composition and application thereof
An anti-tumor drug and composition technology, applied in the field of biopharmaceuticals, can solve problems such as limited gene capacity and hidden dangers of biological safety, and achieve the effects of reduced growth, weak toxicity, and reduced survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
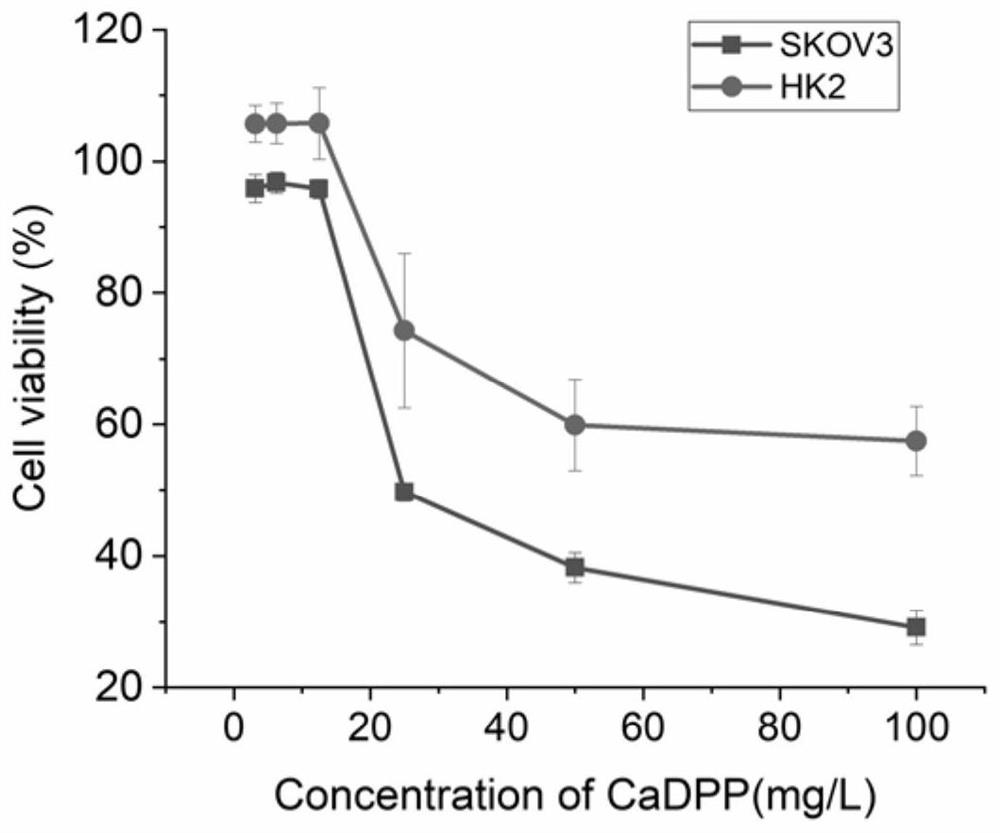
[0035] An anti-tumor pharmaceutical composition, comprising 0.1 mg calcium alendronate and 50 μl anti-EpCAM / CD3 bispecific antibody gene.
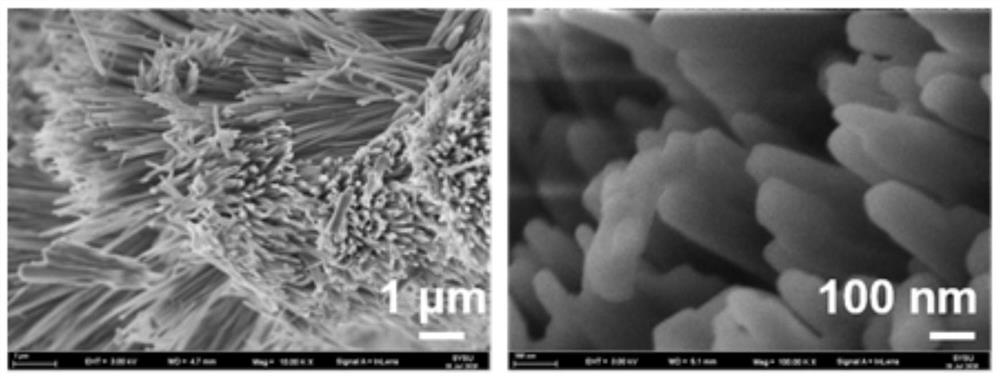
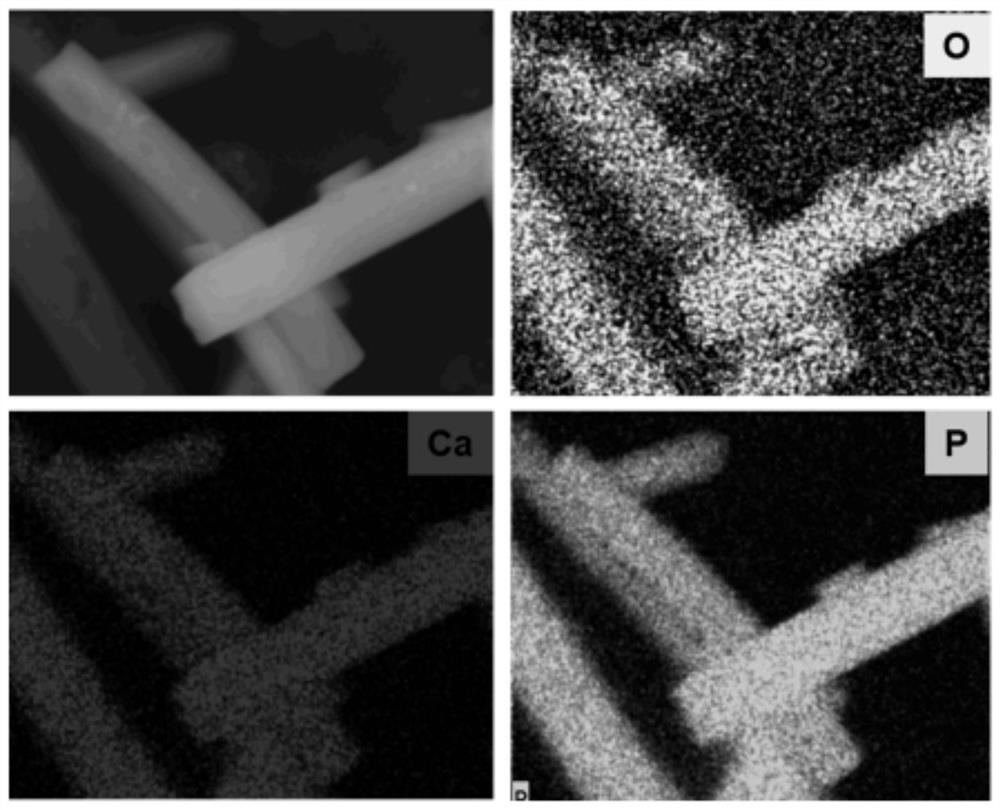
[0036] Wherein the preparation of calcium allen phosphate comprises the following steps: 0.1gCaCl 2 2H 2 O dissolved in 40mL of distilled water to form an aqueous solution of soluble calcium salts, 0.33g sodium alendronate was dissolved in 40mL of distilled water to form an aqueous solution of sodium allenphosphate, and the aqueous solution of soluble calcium salts was added to the aqueous solution of sodium allenphosphate and mixed thoroughly Form a mixture, use NaOH solution to adjust the pH value of the mixture to 5.5, and heat it in a microwave at 100° C. for 10 min to obtain a white crude calcium allenphosphate product; wash the crude calcium allenphosphate product once with distilled water, Washed twice with absolute ethanol, dried in an oven at 60°C for 24 hours to obtain calcium allen phosphate, and dried for later use.
[0037] ...
Embodiment 2
[0039]An anti-tumor pharmaceutical composition, comprising 0.2 mg of calcium alendronate and 50 μl of anti-EpCAM / CD3 bispecific antibody gene.
[0040] Wherein the preparation of calcium allen phosphate comprises the following steps: 0.1gCaCl 2 2H 2 O dissolved in 40mL of distilled water to form an aqueous solution of soluble calcium salt, 0.4g sodium alendronate was dissolved in 40mL of distilled water to form an aqueous solution of sodium alendronate, and the aqueous solution of soluble calcium salt was added to the aqueous solution of sodium allenphosphate and mixed thoroughly Form a mixture, use NaOH solution to adjust the pH value of the mixture to 4.5, microwave heating at a temperature of 80°C for 10 minutes, and obtain a white crude calcium allenphosphate product; wash the crude calcium allenphosphate product once with distilled water , washed twice with absolute ethanol, dried in an oven at 60°C for 24 hours to obtain calcium allen phosphate, and dried for later use....
Embodiment 3
[0043] An anti-tumor pharmaceutical composition, comprising 0.1 mg of calcium alendronate and 60 μl of anti-EpCAM / CD3 bispecific antibody gene.
[0044] Wherein the preparation of calcium allen phosphate comprises the following steps: 0.2gCaCl 2 2H 2 O is dissolved in 40mL distilled water to form an aqueous solution of soluble calcium salt, 0.45g of sodium alendronate is dissolved in 40mL of distilled water to form an aqueous solution of sodium allenphosphate, and the aqueous solution of soluble calcium salt is added to the aqueous solution of sodium allenphosphate and mixed thoroughly Form a mixture, use NaOH solution to adjust the pH value of the mixture to 6.0, microwave heating at a temperature of 95 ° C for 10 min, and obtain a white crude calcium allen phosphate product; wash the crude calcium allen phosphate product once with distilled water , washed twice with absolute ethanol, dried in an oven at 60°C for 24 hours to obtain calcium allen phosphate, and dried for late...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com