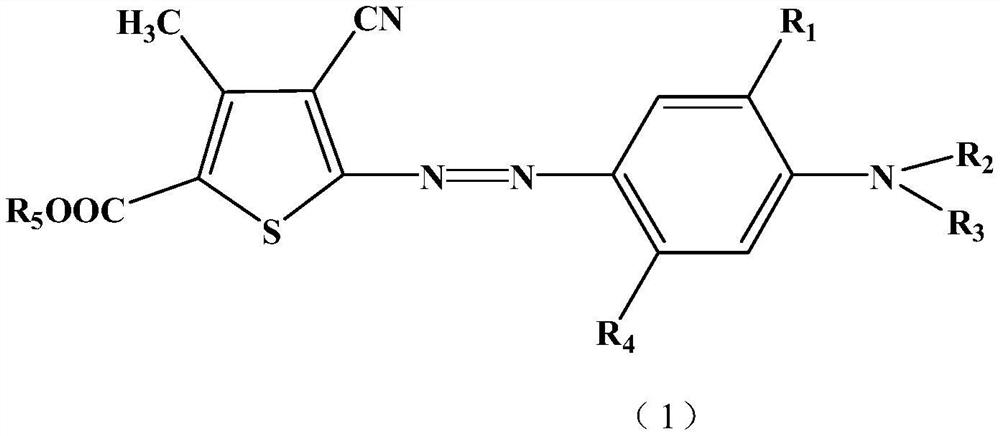
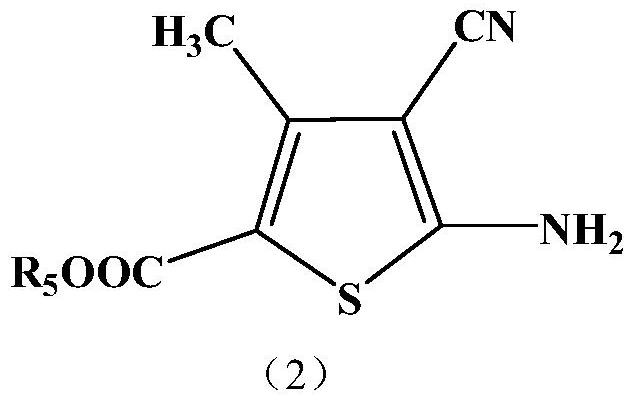
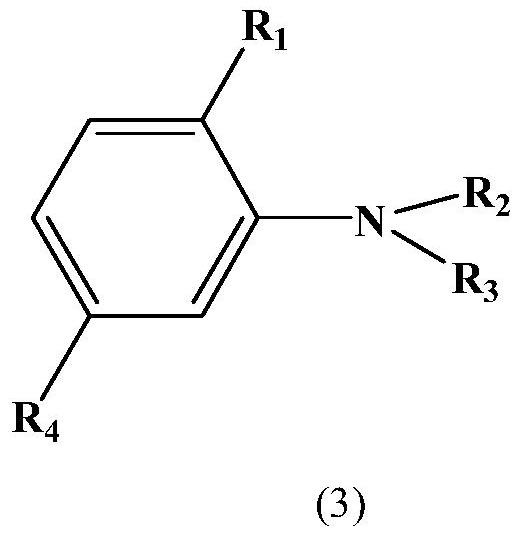
Monoazo compound and preparation method and application thereof
A compound and monoazo technology, applied in the field of monoazo compounds and their preparation, can solve problems such as the decrease of molecular attraction between dyes and fibers, the decrease of fabric fastness, and the decrease of washing fastness, so as to achieve good practical and economic significance , low emissions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The raw materials used in the examples, formula (4) and formula (3a), can be prepared by well-known processes, and other raw materials are commercially available industrial products suitable for dyes and intermediates.
[0060]
[0061] Add 120G of sulfuric acid and 240G of nitrosylsulfuric acid into the flask, cool down to 0-5°C in an ice bath under stirring, add 120G of compound (4) in 1 hour, and stir at this temperature for 3 hours after the addition. After the reaction was completed, drop the diazonium salt into the mixture of 50ML sulfuric acid, 800G ice water and 160G compound (3a) within 1 hour at 0-5°C, continue to stir for 3 hours, filter, wash with water, A dye of the formula is obtained,
[0062]
[0063] The dye is used to dye polyester in bright red light blue. λmax (nm) = 594nm
Embodiment 2
[0065] Add 120G of sulfuric acid and 240G of nitrosylsulfuric acid into the flask, cool down to 0-5°C in an ice bath under stirring, add 120G of compound (4) in 1 hour, and stir at this temperature for 3 hours after the addition. After the reaction was completed, the diazonium salt was dropped into a mixture of 50ML sulfuric acid, 800G ice water and 182G of compound (3b) within 1 hour at 0-5°C,
[0066]
[0067] Continue to stir for 3 hours, filter, wash neutral with water, and obtain the dyestuff of the following formula,
[0068]
[0069] The dye is used to dye polyester in bright red light blue. λmax (nm) = 580nm
Embodiment 3
[0071] Add 120G of sulfuric acid and 240G of nitrosylsulfuric acid into the flask, cool down to 0-5°C in an ice bath under stirring, add 120G of compound (4) in 1 hour, and stir at this temperature for 3 hours after the addition. After the reaction was completed, the diazonium salt was dropped into a mixture of 50ML sulfuric acid, 800G ice water and 198G of compound (3c) within 1 hour at 0-5°C,
[0072]
[0073] Continue to stir for 3 hours, filter, wash neutral with water, and obtain the dyestuff of the following formula,
[0074]
[0075] The dye is used to dye polyester in bright red light blue. λmax (nm) = 585nm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com