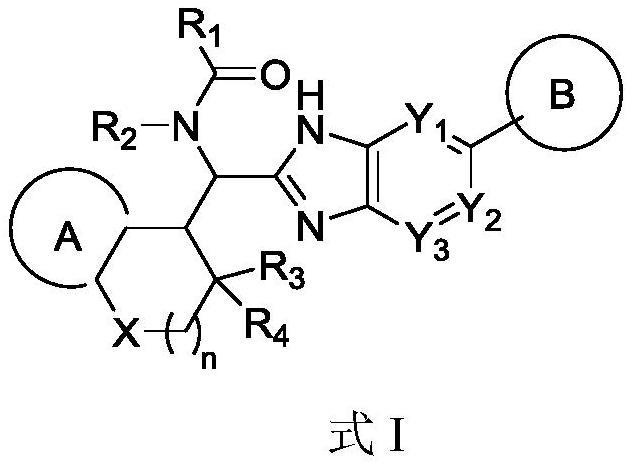
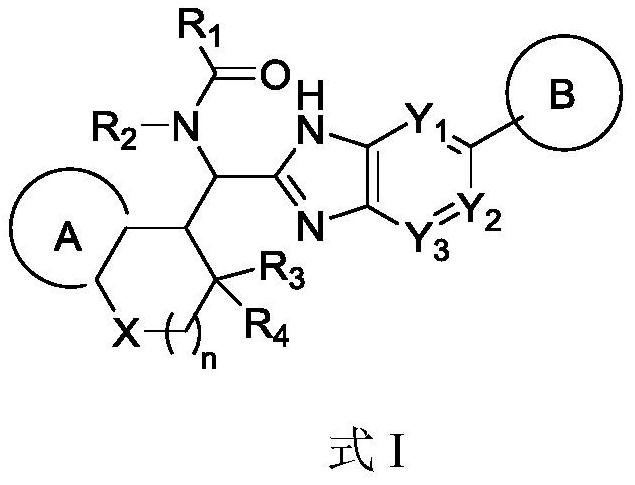
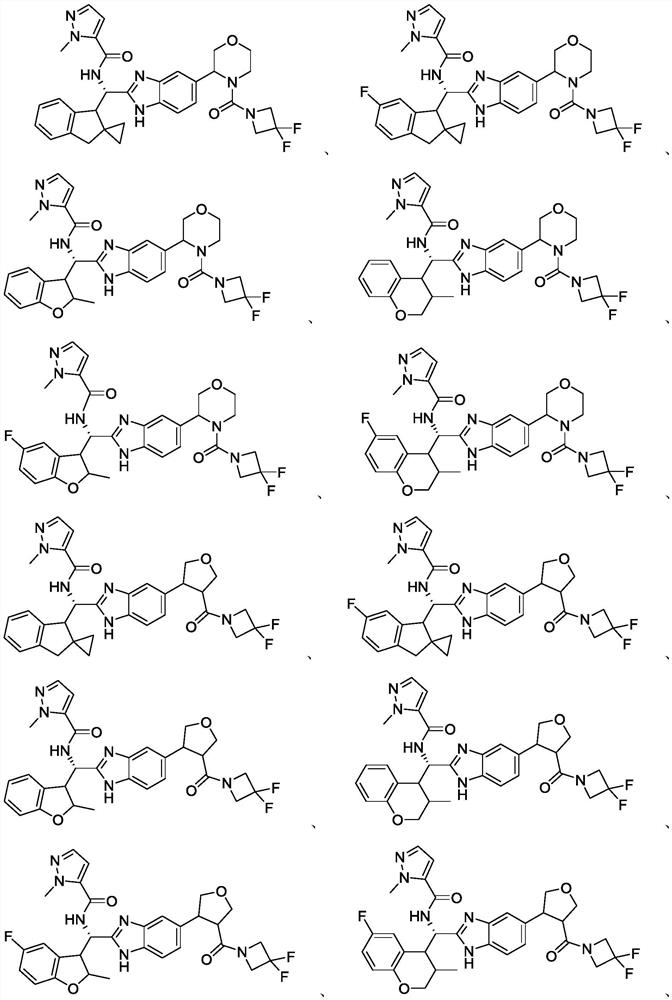
Immunomodulator
A technology of alkylene and alkyl, applied in the field of immunomodulator and its preparation of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The preparation of embodiment 1 intermediate 1
[0115]
[0116] Step 1 Preparation of intermediate 1-1
[0117]
[0118] Dimethyl carbonate (17g, 189mmol) and THF (80mL) were added to a 250mL three-necked flask, added with stirring at room temperature, NaH (60% w / w, 3.18g, 79.4mmol), and protected by nitrogen replacement. A solution of 1-indanone (5 g, 37.8 mmol) in THF (40 mL) was added dropwise to the reaction solution using a dropping funnel. After the dropwise addition, the temperature was raised to reflux for 2 hours, and TLC showed that the reaction was complete. The reaction solution was poured into a mixture of 1M HCl and ice, extracted three times with ethyl acetate (100mL), the combined EA layers were dried, and spin-dried to obtain black oil 1-1 (crude product, 7.11g, yield: 99%) , used directly in the next reaction.
[0119] Step 2 Preparation of intermediate 1-2
[0120]
[0121] Intermediate 1-1 (7.11g, 37.8mmol) was added to a 250mL single-ne...
Embodiment 2
[0146] The preparation of embodiment 2 intermediate 2
[0147]
[0148] The preparation of step 1 intermediate 2-1
[0149]
[0150] Referring to the preparation method of Example 1, dimethyl carbonate (13.5g, 150mmol) and THF (80mL) were added to a 250mL three-necked flask, and NaH (60%w / w, 1.68g, 42mmol) was added under stirring at room temperature, nitrogen Displacement protection. A solution of 6-fluoro-1-indanone (3g, 20mmol) in THF (40mL) was added dropwise to the reaction solution using a dropping funnel. After the addition, the temperature was raised to reflux for 2 hours. TLC showed that the reaction was complete. The reaction solution was poured into a mixture of 1M HCl and ice, extracted three times with EA (100 mL), the combined EA layers were dried, and spin-dried to obtain a black oil 2-1 (4.12 g, crude product), which was directly used in the next reaction.
[0151] Step 2 Preparation of intermediate 2-2
[0152]
[0153] Intermediate 2-1 (4.12g, 20m...
Embodiment 3
[0178] The preparation of embodiment 3 intermediate 3
[0179]
[0180] Preparation of step 1 intermediate 3-1
[0181]
[0182] Referring to the preparation method of Example 1, add ethyl benzofuran-2-carboxylate (5.63g, 29.6mmol) into a 250mL single-necked bottle, add anhydrous THF (60mL) to dissolve, and cool to -78°C in a dry ice-ethanol bath. DIBAL (1M toluene solution, 74mL, 74mmol) was slowly added dropwise, and after the addition was completed, it was slowly raised to room temperature to react overnight, and TLC showed that the reaction was complete. The reaction solution was poured into 1M HCl, stirred at room temperature for 30 minutes, EA (100mL) was added to extract 3 times, the combined EA layers were dried and spin-dried to obtain light yellow oil 3-1 (4.5g, yield: 102.6%), MS m / z:149(M+1) + .
[0183] Step 2 Preparation of intermediate 3-2
[0184]
[0185] Add NBS (5.95g, 33.4mmol) and DCM (80mL) into a 250mL three-necked flask, cool to -30°C under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com