Application of zeartinib in preparation of drugs for treating pulmonary fibrosis diseases
A technology for pulmonary fibrosis and disease, applied in the field of application of zanubrutinib in the preparation of drugs for the treatment of pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
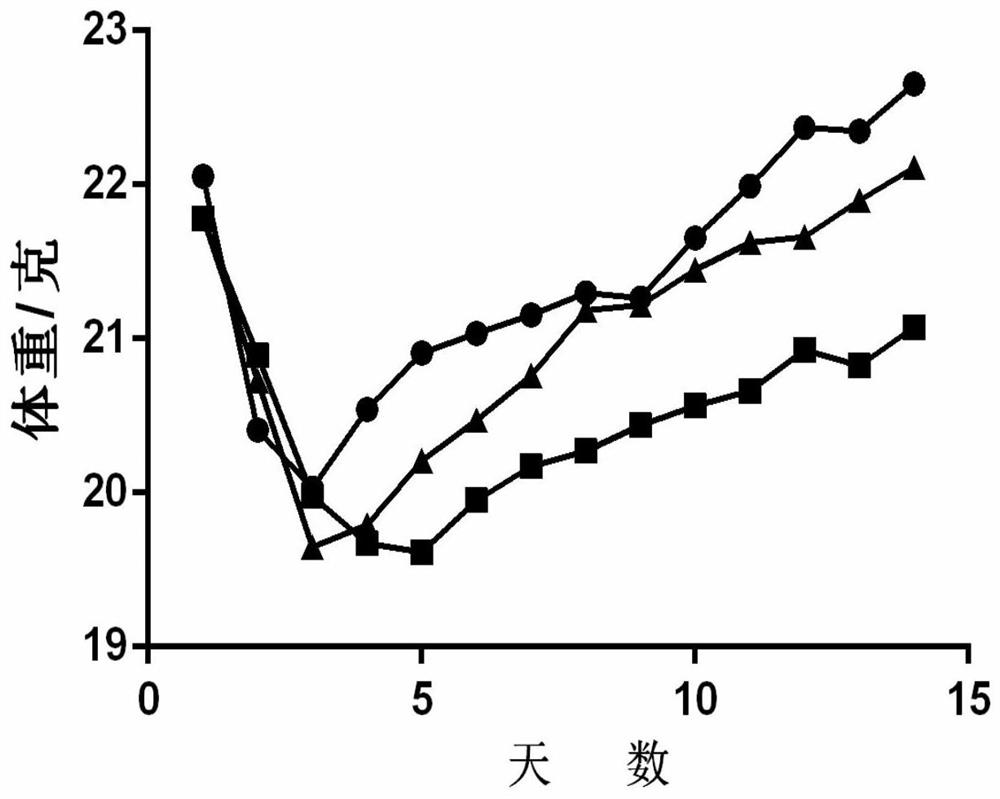
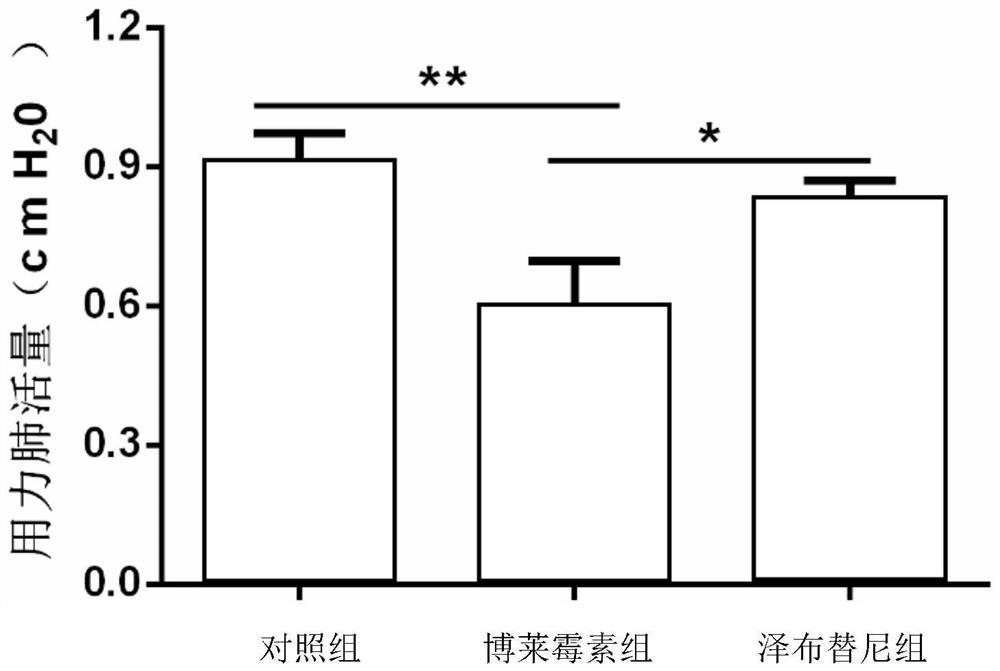
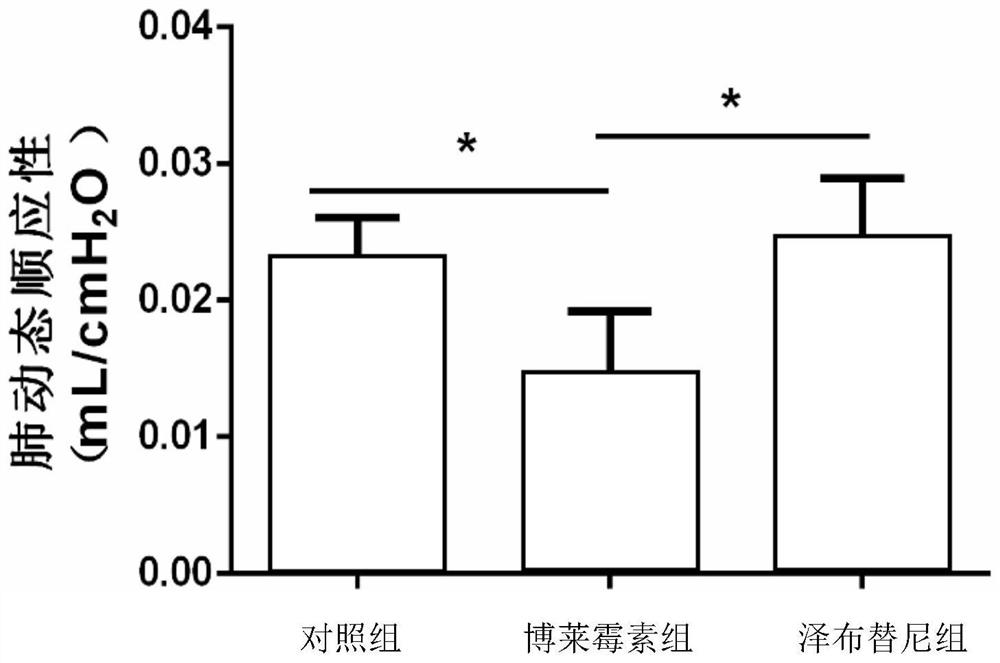
[0029] Example 1: Zanubrutinib slows down bleomycin-induced pulmonary fibrosis in mice
[0030] Animal model preparation: male C57BL / 6J wild-type mice (8-10 weeks old), anesthetized mice, and invasively injected 2U / Kg bleomycin into the trachea.
specific Embodiment approach
[0031] The specific implementation method is as follows: weigh and record after anesthetizing the mouse, fix the mouse on the operating table, disinfect the neck with 75% (v / v) alcohol, and cut a vertical wound of about 1 cm in the neck of the mouse with a scalpel. Use microscopic tweezers to separate the tissues to expose the trachea, insert the syringe into the trachea through the gap between the cartilage rings of the trachea towards the end of the heart, then slowly inject 2 U / kg of bleomycin saline solution in a volume appropriate to its body weight, and immediately put the animal upright And rotate left and right, so that the liquid medicine is evenly distributed in the lungs.
[0032] The blank control group was injected with the same volume of normal saline (0.9% (w / v) NaCl (sodium chloride)).
[0033] Administration in groups: Zenubrutinib treatment refers to giving mice 10 mg / kg of zanubrutinib by intragastric administration every day on the 7th to 14th day of bleomy...
Embodiment 2
[0035] The same as in Example 1, the difference is: in group administration: on the 7th-14th day of bleomycin treatment, mice were given 5 mg / kg zanubrutinib by intragastric administration every day, and the corresponding solvent saline was used as a control , 14 days after bleomycin treatment, lung collagen content and fibrosis severity were detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com