A kind of preparation method of 3β-acetoxy androst-5-ene-17-one
A technology for acetoxyandrostane and androstane, which is applied in the field of organic compound preparation, can solve the problems of low total yield and many by-products, and achieves the effects of cost reduction, convenient operation and novel method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
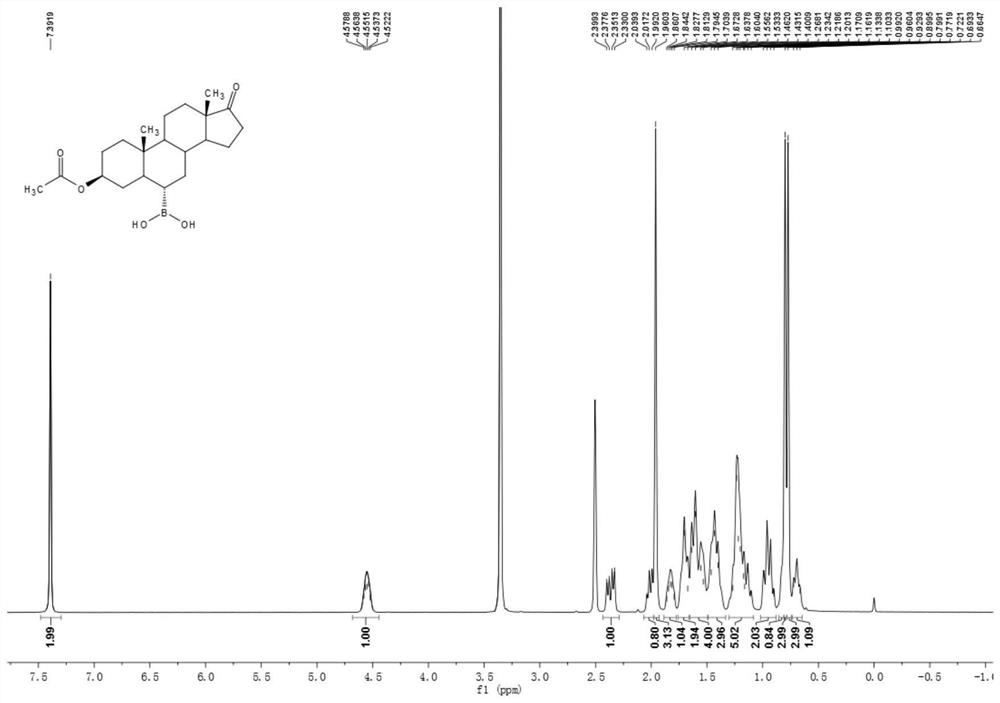
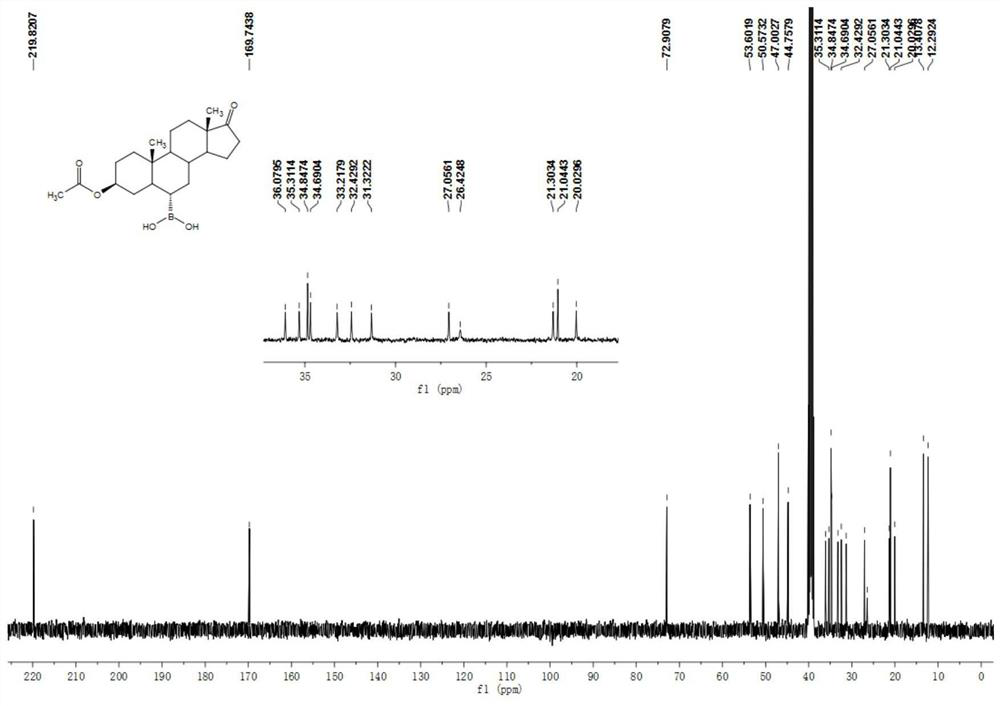
[0050] Example 1 Preparation of 3β-acetoxyandrost-17-keto-6α-boronic acid
[0051] 10g of compound androstane-3β-hydroxy-17-keto-6α-boronic acid, 30ml of acetic anhydride and 10ml of pyridine were added to a 100ml three-necked flask, and the temperature was raised to 80°C under stirring for 1 hour, and the reaction of the raw materials was complete. It was cooled to room temperature, 70 ml of water was added, the solid was precipitated, stirred for 15 minutes, suction filtered, washed with water until neutral, and dried to obtain 11 g of a white solid compound 3β-acetoxyandrost-17-one-6α-boronic acid. 1 H NMR (400MHz, DMSO-d6 ): δ7.39(s, 2H), 4.58-4.52(m, 1H), 2.35(dd, J=19.2, 8.7Hz, 1H), 2.04-1.99(m, 1H), 1.96(s, 3H), 1.86-1.79(m, 1H), 1.71-1.67(m, 2H), 1.64-1.53(m, 4H), 1.46-1.40(m, 3H), 1.27-1.10(m, 5H), 0.99-0.90(m ,2H),0.87-0.81(m,1H),0.80(s,3H),0.77(s,3H),0.72-0.66(m,1H). 13 C NMR (100MHz, DMSO-d 6 ):δ 219.8,169.7,72.9,53.650.6,47.0,44.8,36.1,35.3,34.9,34.7,33.2,32.4,...
Embodiment 2
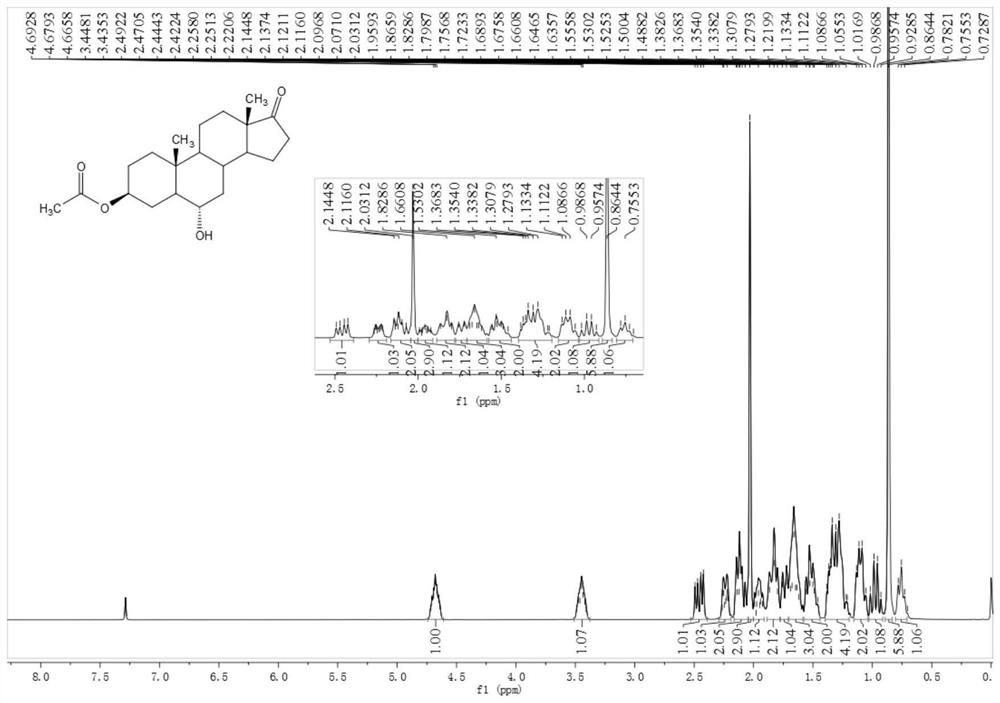
[0052] Example 2 Preparation of 3β-acetoxyandrost-6α-hydroxy-17-one
[0053] Add 11g of compound 3β-acetoxyandrost-17-keto-6α-boronic acid and 25ml of methanol to a 100ml three-necked flask, stir and dissolve, cool to 10°C, add 4g of hydrogen peroxide with a content of 30%, stir for 30 minutes, and heat up to 20°C for reaction After 2 hours, the reaction of the raw materials was completed, and it was quenched with 10% sodium bisulfite. Recover methanol under reduced pressure, add 40 ml of water and 50 ml of dichloromethane, stir and wash for 5 minutes after evaporating to dryness, and then stir and wash for 5 minutes. Methane to obtain 9.7 g of oily substance 3β-acetoxyandrost-6α-hydroxy-17-one. 1 H NMR (400MHz, CDCl 3 ): δ4.71-4.65(m, 1H), 3.51-3.38(m, 1H), 2.46(dd, J=19.2, 8.7Hz, 1H), 2.26-2.21(m, 1H), 2.14-2.10(m ,2H),2.03(s,3H),1.99-1.92(m,1H),1.87-1.80(m,2H),1.76-1.72(m,1H),1.69-1.61(m,3H),1.56-1.46 (m, 2H), 1.38-1.21 (m, 4H), 1.13-1.05 (m, 2H), 1.02-0.93 (m, 1H), 0.8...
Embodiment 3
[0054] Example 3 Preparation of 3β-acetoxyandrost-17-keto-6α-ol p-toluenesulfonate
[0055] 9.7 g of the above-mentioned oily substance 3β-acetoxyandrost-6α-hydroxy-17-one and 30 ml of pyridine were added to a 100 ml three-necked flask, and the mixture was stirred and dissolved. The temperature was lowered to 0° C., 8 g of p-toluenesulfonyl chloride was added, and the reaction was stirred at room temperature for 12 hours, and the reaction was complete. 100 ml of water was added, stirred, the solid was precipitated, suction filtered, washed with water, and dried to obtain 13.0 g of 3β-acetoxyandrost-17-one-6α-alcohol p-toluenesulfonate as a white solid. 1 H NMR (400MHz, CDCl 3 ):δ7.77(d,J=7.8Hz,2H),7.33(d,J=7.8Hz,2H),4.58-4.51(m,1H),4.45-4.39(m,1H),2.47-2.40( m, 4H), 2.19-2.16(m, 1H), 2.10-2.03(m, 1H), 1.99(s, 3H), 1.85-1.78(m, 4H), 1.73-1.60(m, 2H), 1.52- 1.15(m, 8H), 1.08-0.95(m, 2H), 0.84(s, 6H), 0.74-0.69(m, 1H). 13 C NMR (100MHz, CDCl 3 ):δ220.2,170.3,144.6,134.4,129....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com