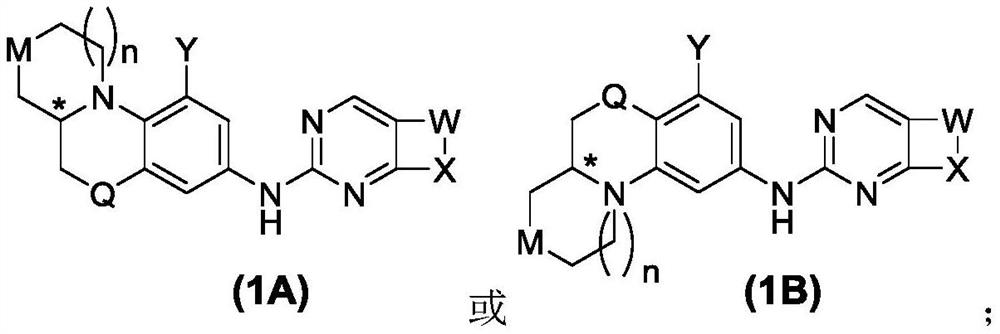
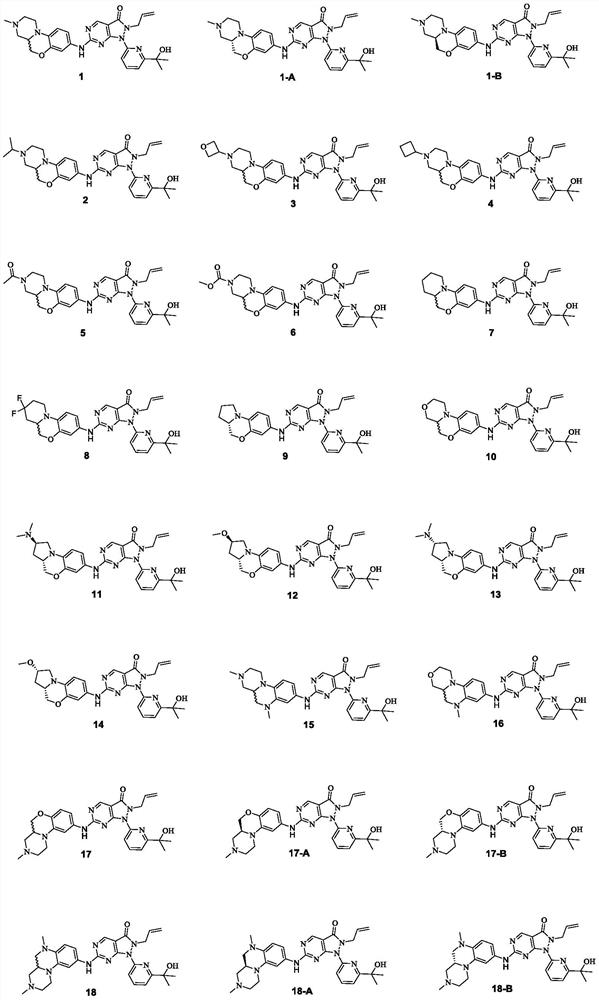
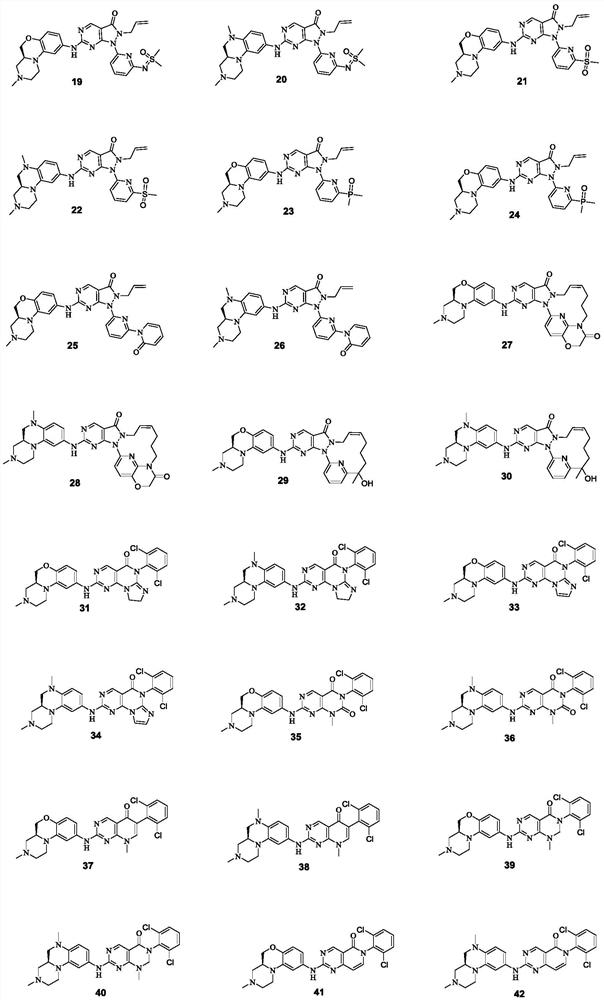
Pyrimidine derivative used as Wee1 inhibitor
A technology of drugs and compounds, applied in the field of anti-tumor drug preparation, can solve the problems of cell apoptosis and DNA damage that cannot be repaired
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Compound 1
[0063]
[0064] synthetic route:
[0065]
[0066] Step 1: Synthesis of compound 1-1
[0067] Dissolve tert-butyl 3-hydroxymethylpiperazine-1-carboxylate (1.0 g, 4.63 mmol) and 1,2-difluoro-4-nitrobenzene (809 mg, 5.1 mmol) in DMF (20 mL), add DIPEA (1.8g, 13.89mmol), heated up to 120°C and reacted overnight. After the reaction was monitored by LC-MS, water (100mL) was added to the reaction system, extracted with EA (50mL*2), the organic phase was combined, and the organic phase was washed with saturated saline ( 50mL) washed with anhydrous Na 2 SO 4 After drying, the filtrate was concentrated and the residue was subjected to column chromatography (PE / EA=10 / 1to 5 / 1) to obtain a yellow solid compound (600 mg, yield 60%), ESI-MS m / z: 356.1 [M+H] + .
[0068] Step 2: Synthesis of Compound 1-2
[0069] Compound 1-1 ((3.6g, 10mmol) was dissolved in DMF (30mL), ice bathed, NaH (60% content, 440mg, 11mmol) was added, and the temperature was ...
Embodiment 2
[0081] Example 2: Compound 2
[0082]
[0083] synthetic route:
[0084]
[0085] Step 1: Synthesis of Compound 2-1
[0086] Dissolve compound 1-3 (706mg, 3.0mmol) in 1,2-dichloroethane (20mL), add acetone (1mL), AcOH (180mg, 3.0mmol), r.t.stir for 1h, add NaBH(OAc) 3 (1.26g, 6mmol), r.t. reacted overnight, LC-MS monitored the completion of the reaction, concentrated, the residue was dissolved in EA (30mL), saturated NaHCO 3 solution (30mL), washed with anhydrous Na 2 SO 4 After drying, the filtrate was concentrated, and the residue was subjected to column chromatography (DCM / MeOH=100 / 1 to 20 / 1) to obtain a yellow solid (400 g, yield 48%), ESI-MS m / z: 278.1 [M+H] + .
[0087] Step 2: Synthesis of compound 2-2
[0088] Compound 2-1 (400mg, 1.44mmol) was dissolved in MeOH (30mL), Pd / C (10%, 80mg) was added, and H 2 , r.t. reacted overnight, LC-MS monitored after the reaction was completed, filtered, and the filtrate was concentrated to obtain a light yellow solid co...
Embodiment 3
[0092] Example 3: Compound 3
[0093]
[0094] Using oxetane and compound 1-3 as raw materials, compound 3 was obtained by using a synthesis method similar to that of Example 2.
[0095] 1 H NMR (400MHz, DMSO-d 6)δ:9.98(s,1H),8.76(s,1H),7.94(t,J=7.9Hz,1H),7.72(d,J=8.1Hz,1H),7.58(dd,J=7.7,0.9 Hz,1H),7.15(d,J=9.3Hz,2H),6.79(d,J=8.9Hz,1H),5.67-5.56(m,1H),5.28(s,1H),4.81-4.73(m ,3H),4.64-4.48(m,4H),4.22(d,J=10.5Hz,1H),3.87-3.70(m,2H),3.64(d,J=11.6Hz,1H),2.96(t, J=9.9Hz, 1H), 2.79(d, J=10.9Hz, 2H), 2.63-2.50(m, 2H), 2.09-2.00(m, 1H), 1.70-1.60(m, 1H), 1.43(s ,6H); ESI-MS m / z:571.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com