Humanized neutralizing active monoclonal antibody against novel coronavirus
A coronavirus and antibody technology, applied in the field of antibody research, can solve problems such as the time needed for clinical application, and achieve the effect of being suitable for transformation application, high clinical value, and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Antigen Preparation
[0080] Cloning, expression and purification of Spike antigen of 2019-nCoV
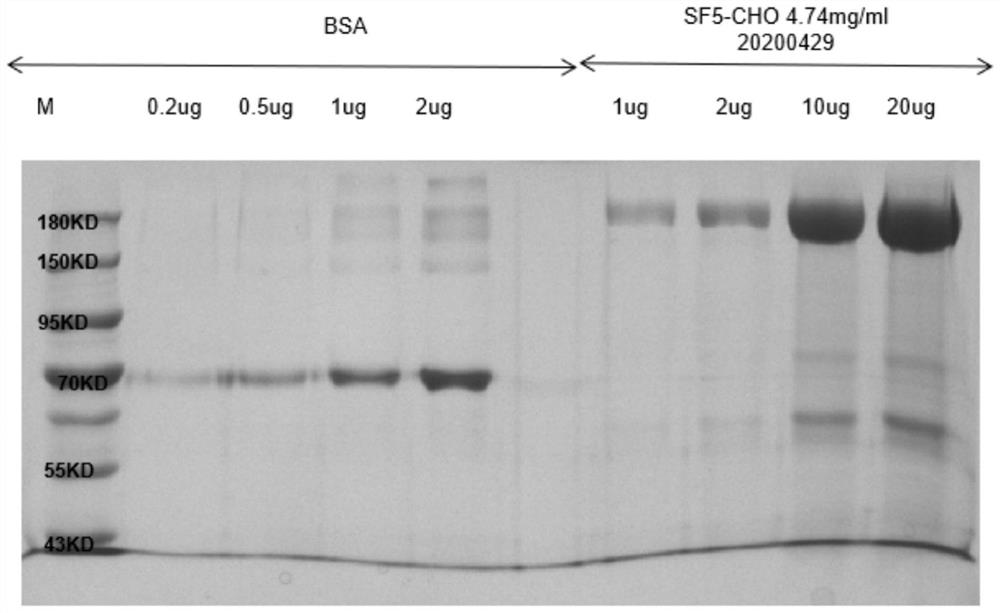
[0081] The sequence of the Spike protein of the new coronavirus was obtained from NCBI, and after codon optimization, it was constructed into the pcDNA3.1 vector. The vector was obtained by Qiagen's EndoFree Plasmid Kit endotoxin-free plasmid extraction kit to obtain a transfectable plasmid, and lipo3000 was used to transiently transfect 293T The cells were transiently expressed at 37 degrees for 7 days, and the cell expression supernatant was harvested, and then affinity purified using a Ni column to obtain the new coronavirus Spike protein with a purity greater than 80%. The purification results are as follows figure 1 shown.
Embodiment 2
[0082] Example 2 Antibody Separation and Screening
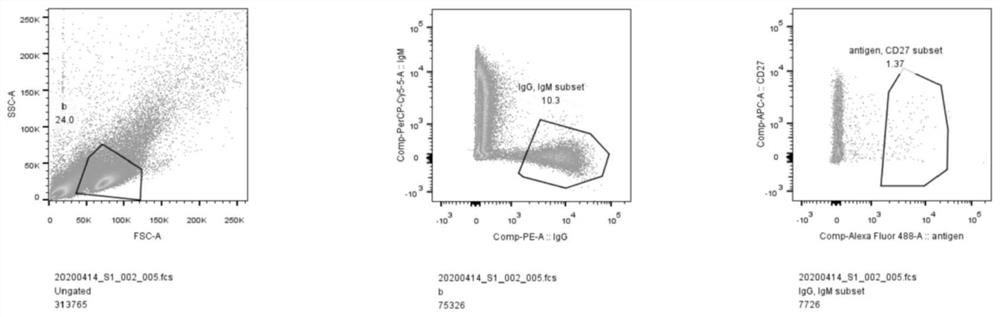
[0083] The prepared Spike antigen-labeled FITC fluorescent dye was purified to obtain S-FITC; peripheral blood lymphocytes were isolated from the peripheral blood of recovered volunteers, and B cells were enriched through the magnetic bead scheme; anti-IgG PE, anti-IgMPerCP- B cells were stained with Cy5.5 and S-FITC, and the IgG+IgM-S+ group new coronavirus S antigen-specific memory B cell subsets were directly sorted into 96 wells that had been added to the cell lysate by flow sorting In a PCR plate, one cell per well.
[0084] Magnetic bead enrichment and sorting single cells into 96-well plates: magnetic bead manufacturer stemcell
[0085] 1. Cell resuscitation: 1 tube each of frozen PBMC cells from recovered patients and PBMC cells from healthy people (cell volume 1*10 7 Cells) were thawed quickly at 37 degrees, and the frozen cells were added to 8ml buffer1 (PBS+2% FBS) preheated at 37 degrees in advance, and centrif...
Embodiment 3
[0101] Example 3 Determination of Antibody Sequence
[0102] By single-cell reverse transcription, the variable region genes of the antibody light chain and heavy chain of a single B cell in each well are transferred.
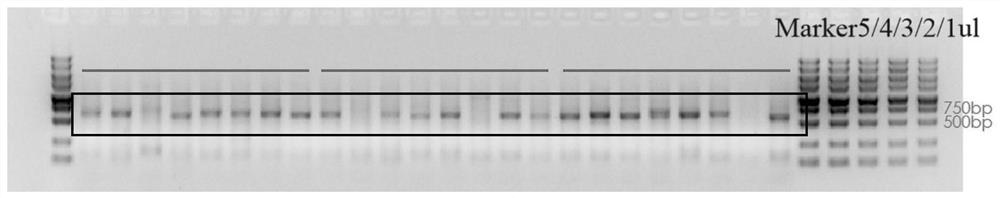
[0103] Single-cell reverse transcription followed the steps of the vazyme N711 kit to obtain double-stranded cDNA, which was used as a template to extract the light and heavy chain genes of a single B cell antibody. System: 25 μl vazyme P515, 2 μl upstream and downstream primers, 1 μl template cDNA , DDW 20μl, the PCR reaction conditions are 95°C, 3min, 95°C, 15s, 60°C, 15s, 72°C, 30s, 72°C, 7min. After gel identification, the positive rate of antibody gene PCR is above 85%.
[0104] image 3 Part of the result of heavy chain gene amplification, Figure 4 Part of the results for light chain gene amplification. Based on this, multiple antibody sequences were obtained, wherein the variable region sequence of the 23D52 antibody gene is shown in SEQ ID NO.7-8, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com