HPLC (High Performance Liquid Chromatography) method for separating naftopidil and enantiomer thereof
A nafidil, volume ratio technology, applied in the field of drug analysis, can solve the problems of manufacturing cost, high sales price, short service life and the like, and achieve the effects of short separation time and reduced separation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]: 1. Instruments and testics
[0022]1, instrument
[0023]US Agilent 1260 Liquid Chromatograph (vacuum degassing, binary pump, automatic injection).
[0024]Columns: Waters Xbridge C18 column (4.6mm × 150 mm, 5 μm).
[0025]Mettler toledo Electronic Balance (XS105).
[0026]2, reagent
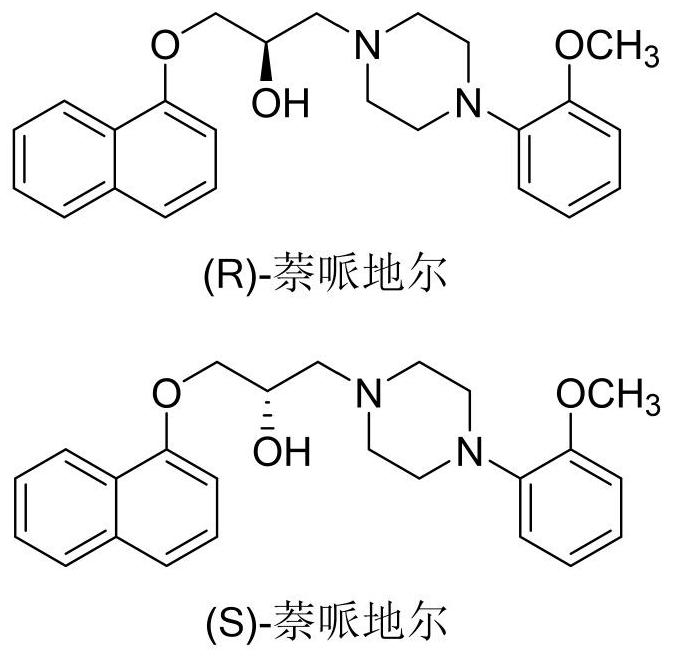
[0027](R) - Napperinini - Part (S) - The purity of the napiulin diolis is not less than 99%, and the structural formula isfigure 1 Indicated.
[0028]Chiral additive FMOC-N-methyl-L-isoleucine is purchased from the microplin reagent, purity 98%.
[0029]Acetonitrile, methanol is pure, water is ultrapure.
[0030]Second, methods and results
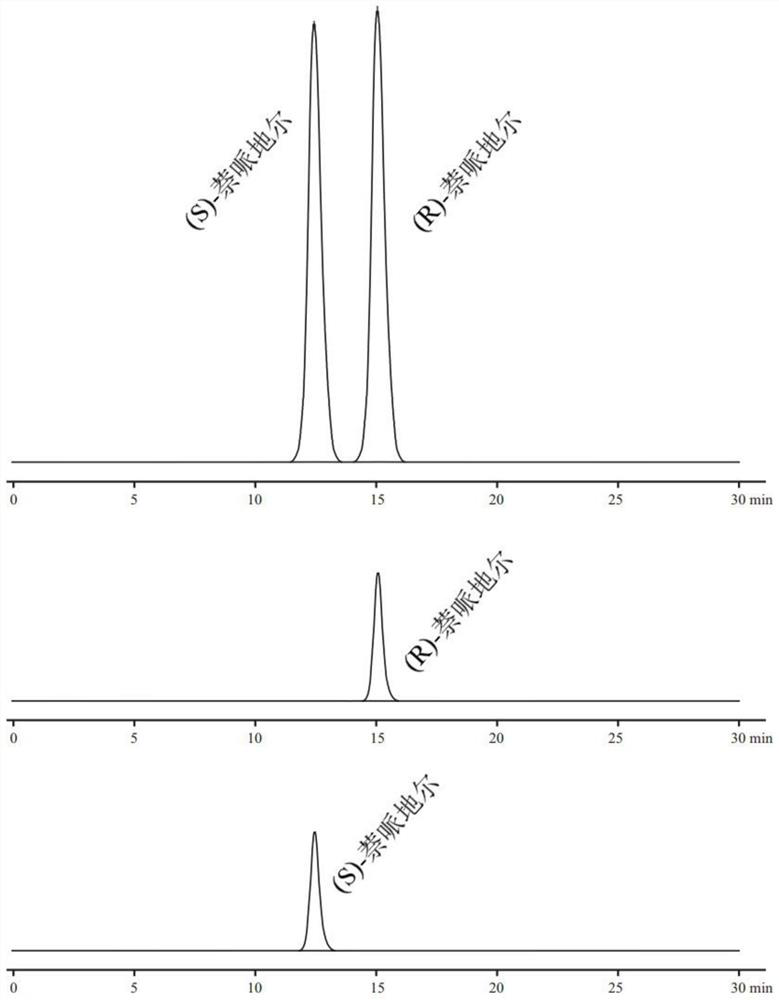
[0031]1, chromatographic conditions
[0032]Column: Waters Xbridge C18 column (4.6mm × 150mm, 5 μm);
[0033]Mobile phase A phase: a 5% aqueous methanol solution containing 0.05 mol / lfmoc-N-methyl-L-isoleucine (formulated method: first mix methanol and water in volume ratio 5:95, then add chiral additive Formulated into desired concentrations);
[0034]Mobile phase B phase: acetonitrile and m...
Embodiment 2
[0046]Example 2: Comparative Example, Mobile Phase does not add a chiral additive
[0047]First, instrument and test
[0048]1, instrument
[0049]US Agilent 1260 Liquid Chromatograph (vacuum degassing, binary pump, automatic injection).
[0050]Columns: Waters Xbridge C18 column (4.6mm × 150 mm, 5 μm).
[0051]Mettler toledo Electronic Balance (XS105).
[0052]2, reagent
[0053](R) - Napperinini - Part (S) - The purity of the napiulin diolis is not less than 99%, and the structural formula isfigure 1 Indicated.
[0054]Acetonitrile, methanol is pure, water is ultrapure.
[0055]Second, methods and results
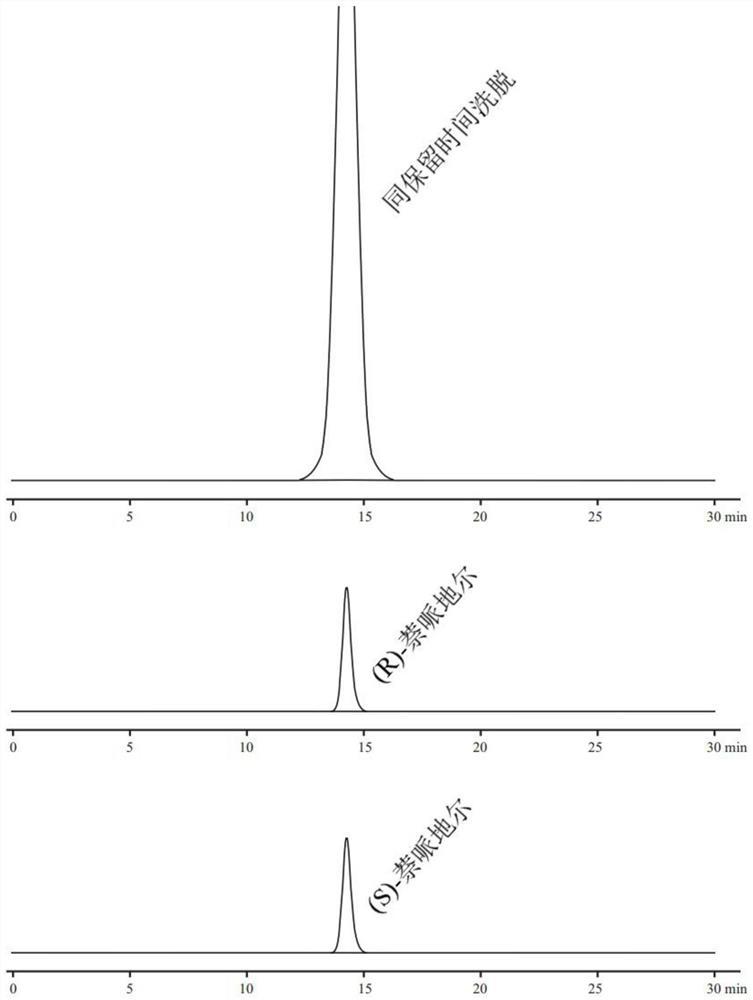
[0056]1, chromatographic conditions
[0057]Column: Waters Xbridge C18 column (4.6mm × 150mm, 5 μm);
[0058]Mobile phase A phase: 5% methanol aqueous solution (preparation method: mixed methanol and water in volume ratio 5:95);
[0059]Mobile phase B phase: acetonitrile and methanol are mixed with a mixed organic solvent formed by volume ratio 4: 3;
[0060]Elip mode and scale: A phase and B phase elute according to v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com