Methods of treating non-syndromic sensorineural hearing loss
A composition and carrier technology, applied in sensory diseases, gene therapy, nervous system cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0297] Example 1: Construction of viral vectors
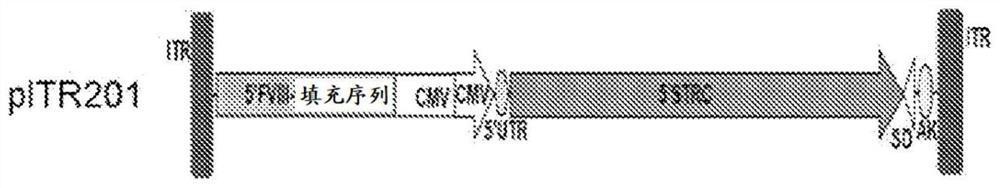
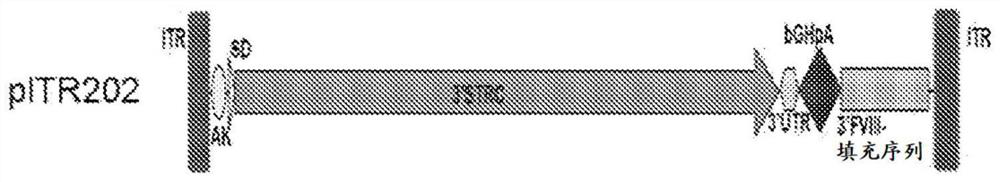
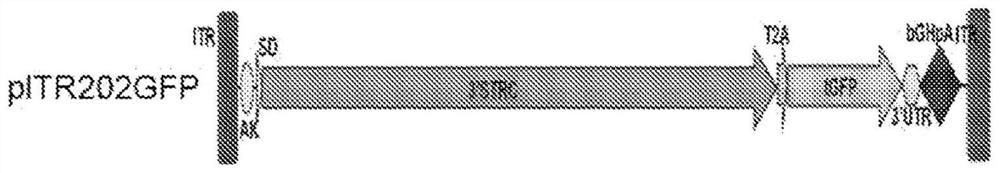
[0298]Recombinant AAV is produced by transfection using an adenovirus-free method as used by Xiao et al. J. Virol. 73(5):3994-4003,1999. A cis-plasmid with AAV ITR, a trans-plasmid with AAV Rep and Cap genes, and a helper plasmid with essential regions from the adenovirus genome were co-transfected in 293 cells at a ratio of 1:1:2. The AAV vectors used here express human sclerostin or mouse sclerostin under various two-vector strategies using the constructs described below. AAV serotypes 1, 2, 3, 4, 5, 6, 7, 8, 9, rh8, rh10, rh39, rh43, and Anc80 were each prepared to encapsulate three sets of sclerostin constructs to test (i) concatenation IL-trans-splicing strategy, (ii) heterozygous intron homologous recombination-trans-splicing strategy, and (iii) exon homologous recombination strategy, as described by Pryadkina et al., Meth.Clin.Devel. 2:15009, 2015 outlined.
Embodiment 2
[0299] Example 2: Production and purification of viral particles
[0300] Recombinant AAV-1 was generated using a triple transfection protocol and purified by two consecutive cesium chloride (CsCl) density gradients as described by Pryadkina et al., Mol. Ther. 2:15009, 2015. At the end of the second centrifugation, 11 500 μl fractions were recovered from the CsCl density gradient tubes and purified by dialysis in IX PBS. Fractions were analyzed by dot blot to determine those that contained the rAAV genome. The number of viral genomes (vg) per preparation was determined by a quantitative real-time PCR-based titration method using primers and probes corresponding to the ITR region of the AAV vector genome (Bartoli et al. Gene. Ther. 13:20-28, 2006).
Embodiment 3
[0301] Example 3: Formulation of Viral Particles
[0302] AAVs produced at a titer of 1e14 vg / mL were prepared in artificial perilymph at dilutions of 3.2e13, 1.0e13, 3.2e12, 1.0e12 vg / mL. Artificial perilymph was prepared by combining the following reagents: NaCl, 120 mM; KCl, 3.5 mM; CaCl2, 1.5 mM; glucose, 5.5 mM; HEPES, 20 mM. The artificial perilymph was titrated with NaOH to adjust its pH to 7.5 (130 mM total Na+ concentration) (Chen et al., J. Controlled Rel. 110:1-19, 2005).
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com