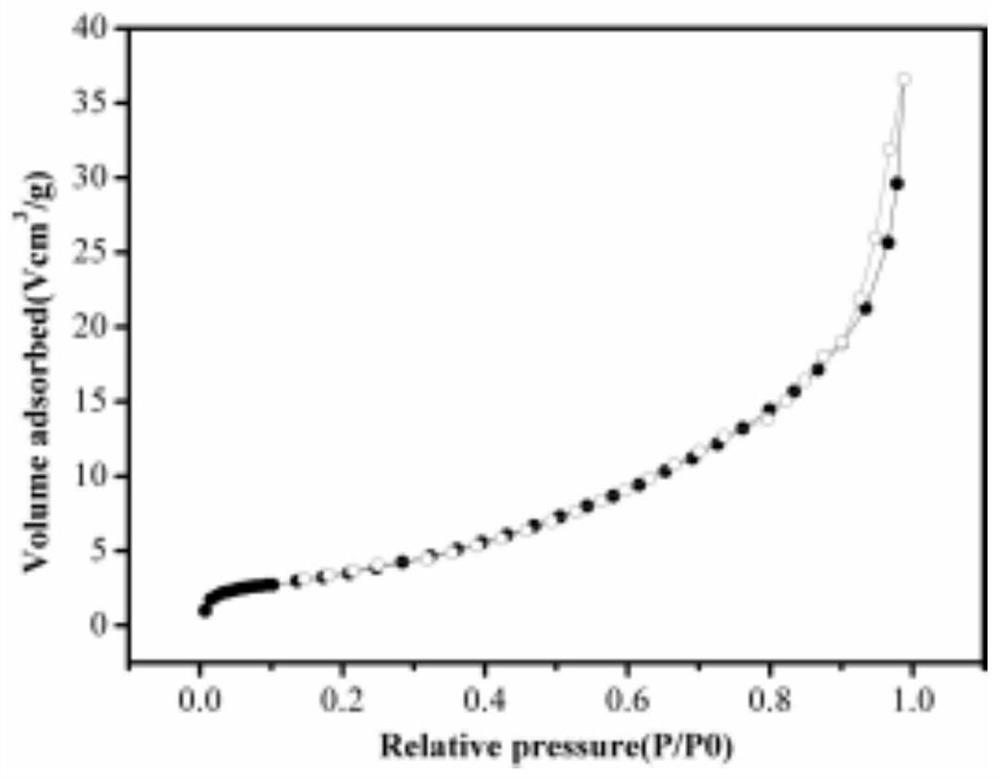
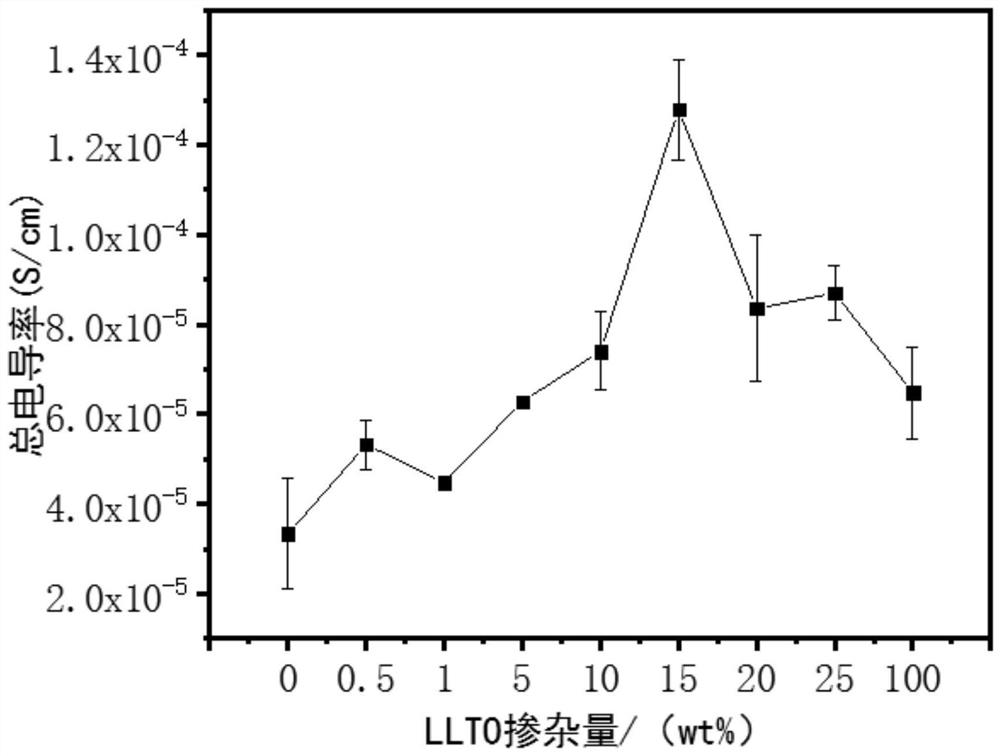
Porous/non-porous composite lithium ion conductor material
A technology of conductor materials and ion conductors, applied in the field of preparation of solid electrolyte materials, can solve problems such as unsatisfactory commercial applications, and achieve the effect of improving total conductivity and high lithium ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] According to the chemical formula Li 0.33 La 0.56 TiO 3 Determination of raw material LiOH·H by stoichiometric ratio 2 O (of which LiOH·H 2 O weighed 10% more to compensate for the loss of lithium at high temperature), La(NO 3 ) 3 ·6H 2 O, butyl titanate, CTAB. Dissolve CTAB in deionized water, then add La(NO 3 ) 3 ·6H 2 Stir at room temperature for 30min, dissolve butyl titanate in isopropanol and slowly add it dropwise into the above nitrate solution, stir for 45min, then add LiOH·H 2 O, stirred for 1h and then transferred to the reactor, and reacted at 180°C for 36h. Take it out and dry it, place it in a box-type muffle furnace to raise the temperature to 500°C at a rate of 5°C / min, keep it warm for 4 hours, then raise the temperature to 800°C, keep it warm for 2 hours, take it out and grind it fully, and then use a tablet press to form it. In the muffle furnace, the temperature was raised to 800°C at a rate of 5°C / min, kept for 2 hours, then raised to 135...
Embodiment 2
[0022] According to the chemical formula Li 0.33 La 0.56 TiO 3 Determination of raw material LiOH·H by stoichiometric ratio 2 O (of which LiOH·H 2 O weighed 10% more to compensate for the loss of lithium at high temperature), La(NO 3 ) 3 ·6H 2 O, butyl titanate. Take La(NO 3 ) 3 ·6H 2 O was dissolved in deionized water, stirred at room temperature for 30 minutes, dissolved butyl titanate in isopropanol and slowly added to the above nitrate solution, stirred for 45 minutes, and then added LiOH·H 2 O, stirred for 1h and then transferred to the reactor, and reacted at 180°C for 36h. Take it out and dry it, place it in a box-type muffle furnace to raise the temperature to 500°C at a rate of 5°C / min, keep it warm for 4 hours, then raise the temperature to 800°C, keep it warm for 2 hours, take it out and grind it fully, and then use a tablet press to form it. In the muffle furnace, the temperature was raised to 800°C at a rate of 5°C / min, kept for 2 hours, then raised to ...
Embodiment 3
[0025] According to the chemical formula Li 0.33 La 0.56 TiO 3 Determination of raw material LiOH·H by stoichiometric ratio 2 O (of which LiOH·H 2 O weighed 10% more to compensate for the loss of lithium at high temperature), La(NO 3 ) 3 ·6H 2 O, butyl titanate, CTAB. Dissolve CTAB in deionized water, add 0.5wt% non-porous LLTO lithium ion conductor powder, stir at room temperature for 1 h, add La(NO 3 ) 3 ·6H 2 Stir at room temperature for 30min, dissolve butyl titanate in isopropanol and slowly add it dropwise into the above nitrate solution, stir for 45min, then add LiOH·H 2 O, stirred for 1h and then transferred to the reactor, and reacted at 180°C for 36h. Take it out and dry it, place it in a box-type muffle furnace to raise the temperature to 500°C at a rate of 5°C / min, keep it warm for 4 hours, then raise the temperature to 800°C, keep it warm for 2 hours, take it out and grind it fully, and then use a tablet press to form it. In the muffle furnace, the temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com