SiO<2> coated Co<2+>-Cu<2+> doped amorphous nickel nitrate lithium battery negative electrode material and preparation method thereof
A technology of lithium nickel nitrate and negative electrode material, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as rate performance, difficulty in maintaining cycle capacity, affecting battery performance, dissolution, etc., to improve comprehensive electrochemical performance, improve Lithium ion conductivity, the effect of extending diffusion migration channels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
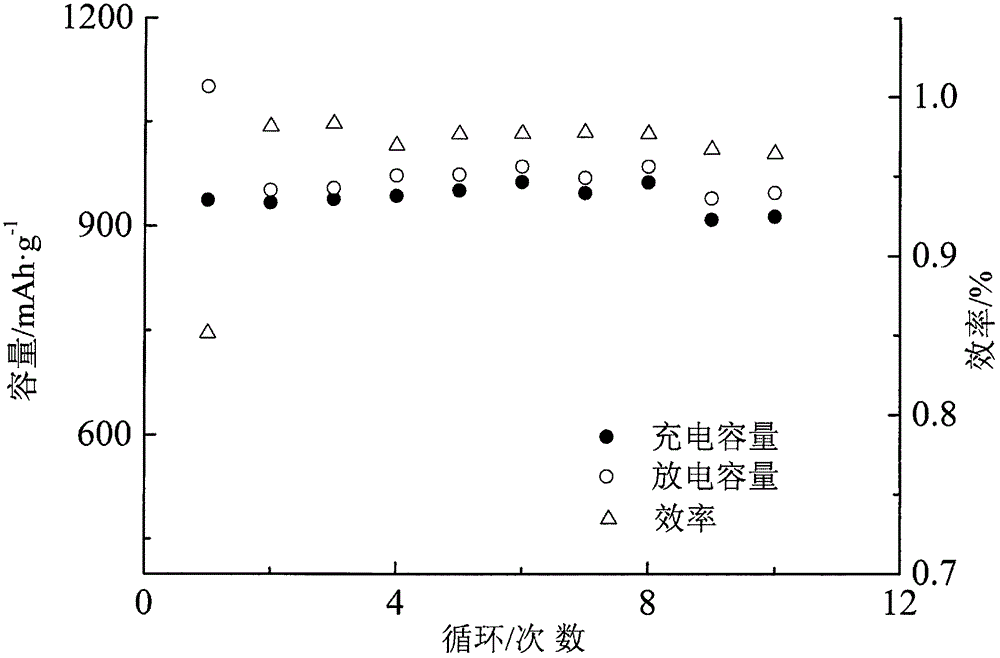
Image
Examples
Embodiment 1
[0013] Embodiment 1: the cobalt nitrate hexahydrate of nickel nitrate hexahydrate, the amount of 0.5% copper nitrate hexahydrate nickel nitrate substance, the amount of 0.5% of nickel nitrate hexahydrate substance are dissolved in deionized water to form a total metal ion concentration of 0.5mol·L -1 The solution; This solution of equal mass is mixed with hexanaphthene, is added the CTAB that the liquid mass percentage after mixing is 0.5%, the n-butanol that the liquid mass percentage after mixing is 0.1%, stirs 5 minutes with the rotating speed of 500 rev / min After standing still for 5 minutes, then add 0.5% tetraethyl orthosilicate in the amount of nickel nitrate hexahydrate and stir at a speed of 120 rpm for 2 minutes and then let stand for 3 hours; min -1 The speed is passed into the injection port of the spray dryer through the peristaltic pump, and other operating conditions are: the air intake is 0.5m 3 min -1 , The air inlet temperature is 100°C, and the air outlet...
Embodiment 2
[0014] Embodiment 2: the copper nitrate trihydrate of the amount 5% of nickel nitrate hexahydrate, nickel nitrate hexahydrate, the cobalt nitrate hexahydrate of the amount of nickel nitrate hexahydrate 5% are dissolved in deionized water to form a total metal ion concentration of 1.5mol·L -1 The solution; This solution of equal mass is mixed with hexanaphthene, is added the CTAB that the liquid mass percentage after mixing is 3.0%, the n-butanol that the liquid mass percentage after mixing is 1.0%, stirs 15 minutes with the rotating speed of 900 rev / min After standing still for 10 minutes, then add tetraethyl orthosilicate with the amount of nickel nitrate hexahydrate 5% while stirring at a speed of 200 rpm for 5 minutes and then let stand for 10 hours; min -1The speed is passed into the injection port of the spray dryer through the peristaltic pump, and other operating conditions are: the air intake volume is 3.5m 3 min -1 , The air inlet temperature is 130°C, and the air ...
Embodiment 3
[0015] Embodiment 3: nickel nitrate hexahydrate, nickel nitrate hexahydrate amount 3% copper nitrate trihydrate, nickel nitrate hexahydrate amount 3% cobalt nitrate hexahydrate are dissolved in deionized water to form a total metal ion concentration of 1.0mol·L -1 The solution; This solution of equal mass is mixed with hexanaphthene, is added the CTAB that liquid mass percentage is 1.0%, the n-butanol that liquid mass percentage is 0.5% after mixing, stirs 10 minutes with the rotating speed of 600 rev / min After standing still for 8 minutes, then add tetraethyl orthosilicate in an amount of 3% nickel nitrate hexahydrate while stirring at a speed of 160 rpm for 4 minutes and then let stand for 6 hours; min -1 The speed is passed into the sample inlet of the spray dryer through the peristaltic pump, and other operating conditions are: the air intake is 2.5m 3 min -1 , The temperature of the air inlet is 120°C, and the temperature of the air outlet is 85°C; the collected solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com